INTRODUCTION
Food can be the source of a broad range of chemical contaminants and residues of agrochemicals. Some may be present naturally, or they may occur as a result of contamination or processing, or they also may be applied by the agriculture or manufacturing industry for their functional properties. Sometimes, chemicals are also added for malicious reasons, e.g., economic fraud, tampering or terrorism. Thus, considering the plethora of chemical hazards that may be present in food, a risk-based approach for the management of these is usually needed. The HACCP system, a risk-based approach to food safety assurance, was originally developed to manage the safety of microbiological hazards in the food supply. But it is recognized that the principles of the system can also be used for the management of chemical contaminants. This article describes the management of food chemical contaminants, based on
HACCP principles. However, it is to be noted that the application of HACCP may not be the
only approach. In any case it should ideally be based on (1) a conscious and proactive analysis of potential hazards – in particular those for which there are regulatory limits, (2) the analysis of their risk, based on sound scientific evidence, (3) setting in place effective measures to prevent or control their occurrence within agreed acceptable limits, and (4) verifying that the food safety management system is effective.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
NATURE OF CHEMICAL HAZARDS
Chemical hazards1 can be broadly categorized as follows:
● Environmental contaminants: originate from the environment (soil, air, water), either naturally or as a result of anthropogenic activity. They are present in/on the raw material and they enter into the product in this way. Examples are toxic metals (cadmium, lead, mercury, arsenic and aluminum), polychlorinated biphenyls (PCBs), dioxins and radionuclides.
● Naturally occurring toxins: are produced naturally by plants, algae, fungi or marine organisms. Examples include: plant toxicants (e.g., solanine in potatoes), mycotoxins (e.g., aflatoxins), marine biotoxins (e.g., saxitoxin responsible for paralytic shellfish poisoning). Although some foodborne pathogens also produce toxins, they are often addressed in the context of microbial food safety management.
● Processing contaminants: are undesirable compounds that are formed during the treatment of food as the result of the interaction of its components. Examples are acrylamide, chloropropanols, furan, benzene, ethyl carbamate.
● Packaging contaminants: are components of packaging material or ink, which then migrate into the product. Examples are Bisphenol A diglyceryl ether (BADGE), phthalates and epoxidized soybean oil (ESBO). They are sometimes grouped under “surface contact contaminants.”
● Food additives: certain food additives, when present in high levels in food, may present a health risk. An example is nitrate. In the scope of this text, only food additives that have an established ADI are considered as “potential hazards.”
● Agrochemicals: include veterinary drugs and pesticides. Similar to food additives, agrochemicals are considered as a hazard if they occur at levels above regulatory limits or internally safety-based norms.
Additionally, foods may be subject to:
● Accidental contamination from various chemical agents used for manufacturing purposes; examples are disinfectants, cleaning agents and lubricants.
● Adulteration, e.g., use of unauthorized substances such as unauthorized dyes. This is often practiced for economic reasons.
● Terrorism or sabotage. These are often deliberately added to food for malicious reasons.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
HEALTH CONSEQUENCES
It is well established that the health consequences of chemical hazards depend on three factors:
1. Nature of the agent.
2. Amount present in the food and the intake of consumers.
3. Vulnerability of consumers.
The health effects vary according to the dose. As Paracelsus (Swiss physician and chemist, 1494–1541) stated, “All things are poison, and nothing is without poison; only the dose permits something not to be poisonous,” or, more concisely, “The dose makes the poison.” At high doses, chemical hazards can lead to acute or fatal intoxication, or allergic reaction in the case of allergens. Upon long-term exposure at low doses, they can also cause adverse health conditions and be a risk factor for various chronic diseases. For a thorough overview of the health risks associated with chemicals, the reader is referred to Moy and Todd (in press).
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
FACTORS AFFECTING THE OCCURRENCE OF CHEMICAL HAZARDS
Depending on the nature and the source of chemical hazards, different factors may influence their occurrence in the raw material or during processing. Understanding these factors and their consideration in the hazard analysis is essential for evaluating the likelihood of occurrence and deciding on appropriate control measures and verification activities. Examples of such factors are:
● Agronomical
● General farm/agricultural practices (e.g., conventional, contract, bio).
● Disease in animals/plants.
● Nature of soil.
● Price and availability of agrochemicals (e.g., easy access to unapproved or banned agrochemicals).
● Climatic
● Climatic fluctuations may stress plants and promote fungal attacks, which increase the risk either of mycotoxins or of abuse when using agrochemicals.
● Stress caused by drought or excessive rain increase the risk of pre-harvest mold growth. Droughts have also led to feeding cattle with plants not intended as feed, thus contaminating unapproved agrochemicals.
● Insect infestations also stress/damage plant tissues and increase the risk of mold
growth and subsequent mycotoxin formation.
● Environmental
● Industrial activity and pollution can lead to contamination of soil, atmosphere and water with chemical hazards such as heavy metals or dioxins.
● Soil may also naturally contain high levels of certain chemical agents, such as heavy metals and POPs (persistent organic pollutants). Mining activities can also increase exposure to toxic metals.
● Suppliers’ practices
● Suppliers’ farm or agricultural practices.
● Manufacturing practices and method of processing.
● Suppliers’ QA system (preventive measures, monitoring measures).
● Legislation
● Regulatory requirements, i.e., if a country lacks appropriate legislation.
● Enforcement
● If the authorities are not enforcing and monitoring the implementation of the legislation.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
REGULATORY REQUIREMENTS AND CHALLENGES
To protect consumers’ health and ensure the safety of the food supply, public health authorities establish maximum limits for various contaminants, maximum levels of use for food additives and maximum residues limits (MRLs) for veterinary drugs and pesticides. Specific migration limits are also established for various packaging contaminants. One of the fundamental considerations in setting standards for chemicals is the health effects of chemicals, from the perspective of both short-term and long-term exposure. At the national level, regulatory standards, or norms, are generally established based on
the consideration of the health risk associated with a given chemical, but also taking into account other factors such as feasibility to comply, nutritional needs and the diet of the population. Therefore, regulatory standards are often a trade-off between the health risk of a chemical and what is achievable and appropriate for society. As such, it is a risk management decision. Nevertheless, regulatory standards established for a chemical hazard are viewed by society as a food safety standard and industry has the obligation to abide by these standards. With respect to chemical hazards, exceeding these standards must be seen as a violation of food safety at the international level, the World Health Organization (WHO) and the Food and Agriculture Organization of the United Nations (FAO) sponsor the Joint Expert Committee
on Food Additives and Contaminants (JECFA) and the Joint Meeting on Pesticide Residues (JMPR), which carry out risk assessment of chemical contaminants, food additives, veterinary drugs and pesticides, respectively. Based on these risks assessments, the FAO/WHO Codex Alimentarius Commission (CAC) establishes international standards for food. Since the establishment of the World Trade Organization (WTO) and the coming into force of the Agreement on the Sanitary and Phytosanitary Measures in 1995, the work of CAC, e.g., its standards, have become the international reference for food safety. This means that products that comply with Codex standards cannot be rejected on food safety grounds by the WTO member states unless the importing country provides scientific evidence (based on risk assessment) that the product in question is not appropriate for its population.
REGULATORY COMPLIANCE
The food industry has the obligation to comply with all the laws and regulations of the country in which they market the food. Considering that some countries may have different standards, multinational companies will have to produce foods of different standards. This raises the issue of double standards, or even that of dumping of foods of higher level of contamination in countries with lower standards. For ethical reasons, it is thus recommended that multinational companies meet the Codex Alimentarius standards as a minimum; it must be noted that the Codex standards are today recognized as the internationally agreed requirements for food safety. Where national or international requirements are not established, the food industry still has the obligation to produce safe food; thus, in some cases, internal norms may be needed on the basis of the due diligence principle.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
MANAGEMENT OF CHEMICALS IN INDUSTRY Prerequisites
HACCP is applied in conjunction with a number of supporting measures, which are generally referred to as prerequisites. The term is used to emphasize that the HACCP system is not a stand-alone system and that its successful implementation depends on a number of programs embedded in the food safety management system such as (1) good agricultural practice (GAP), good animal husbandry practice (GAHP) or good manufacturing practice (GMP), (2) supplier or vendor confidence level and (3) personnel training (including managers, supervisors, shop floor operators, technicians and laboratory personnel).
As most of the chemical contaminants in products come with the raw material and, once present, generally cannot be removed, supplier/vendor management is a key prerequisite in food businesses. Due to its importance, some key guidance is provided below. Additionally, there are other “measures” or requirements which are not usually referred to as prerequisites, but which in practice are the conditio sine qua nons for the management of chemical hazards. Therefore, they deserve to be mentioned here. These are:
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
● Scientific knowledge (e.g., understanding the mechanism of formation of processing contaminants, conditions for growth of molds, impact of control measures, etc.).
● Legislation (e.g., norms, codes of practices) and enforcement.
Where these measures are not in place, the likelihood of a contaminant being present or occurring is higher. Therefore, before conducting a hazard analysis, the implementation of the above needs to be evaluated, and in case of gaps, their application needs to be improved in the first place. In the interim, the risks which may ensue from the gaps in prerequisite programs need to be considered in the hazard analysis, and the chemical in question must be considered as potentially significant. For instance, during import of a raw material from a region or country where the legislation related to the use of veterinary drugs or pesticides is not established or enforced, the likelihood of the presence of unauthorized residues in the commodity, marketed in the selling country above safe or regulatory levels, must be considered likely.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
Supplier Management
Considering that many chemical hazards are introduced into products through the raw material, the importance of supplier management cannot be overemphasized. Supplier management starts by selecting the supplier. However, before doing this, there is a need to understand the suppliers’ expectations and whether they will be capable of producing the material according to specifications. Therefore, the process starts with understanding the requirements, the quality and safety objectives and formulating the
specifications.
Specifications
A specification is a description of a material’s properties and values (e.g., physical, chemical, sensorial, microbiological, as well as transportation and storage requirements). One may differentiate between purchasing specifications and finished product specifications. Purchasing specifications is an important instrument to convey to suppliers the requirements in terms of food safety and quality. As such, chemical contaminants that are likely to be present in the raw material at an unacceptable level must be prescribed.
The requirements to be mentioned in the purchasing specifications must follow the hazard analysis during the HACCP study, taking into account the conditions of production or manufacturing of the raw or packaging material. In preparing the specifications, consultation of the supplier is recommended since the supplier will have specific expertise on the subject. The regulatory requirements of the country where the product is manufactured and/or sold are also important when establishing the specification.
For unauthorized compounds, the specification must indicate “absence.” The minimum performance criterion of the analytical method2 expressed as limit of detection (LoD) and/or limit of quantification (LoQ) has to be given in this case. The units of the limits should be expressed according to SI norms (e.g., mg/kg, ng/l). Finished product specification relates to norms for the final product and is especially
important for certain types of contaminants and products, such as products that constitute
an important part of the diet. The finished product specification must conform to the regulatory requirements of the country where the product is sold and/or with the CAC norms,
whichever is stricter. The finished product specification represents the final consolidation of
all the requirements, be they regulatory, safety or quality related, and it is the key document
for compliance verification.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
Selection of the Supplier
When selecting and approving a supplier, consideration must be given to the supplier’s ability to meet the purchasing specifications, in particular the supplier’s:
● Awareness of chemical hazards associated with their products.
● Consideration of regulatory requirements in their HACCP studies.
● Raw material and management of their own supply chain.
● Traceability.
● Implementation of control measures at the CCPs.
● Practices with regard to the processing and storage of raw material and semi-finished or
finished products.
● Monitoring activities and records.
● Training program for personnel as well as suppliers’ laboratory capabilities and
performance.
Certificate of Analysis (CoA)
As a confirmation of the suppliers’ compliance with the requirements, a CoA may be required. The CoA is to be viewed as a verification of control measures at the suppliers’ level. It is thus a complement to internal monitoring. However, care must be taken that the CoA is provided by a competent accredited or approved laboratory. In absence of accreditation, a periodic independent or in-house verification of the CoA is necessary. Alternatively, suppliers may provide a certificate of compliance (CoC). This is different from a CoA. It is basically a certificate stating that the material complies with the requirements, including compliance with the regulatory requirements or recognized international standards. It is to be noted that a CoC is not based on the analytical results, and that its validity depends on the measures that the supplier puts in place to meet the set requirements. This has to be verified during audits of suppliers.
In the delivery of the certificate, the following conditions must be respected:
● The certificate must refer to an actual analysis of the lot being delivered, not to an average monthly sample, or to a previously analyzed lot. It must cover all the parameters agreed with the supplier.
● The sampling method and sampling plan must be mutually agreed upon.
● The laboratory carrying out the analysis must be clearly identifiable on the certificate.
● The report must identify the analytical methods used.
● The accuracy of results for chemical parameters must be verified periodically
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
Analytical Aspects
Besides the analytical performance of a test method that is used to analyze a specific
chemical hazard, the manner of reporting test results may also have an impact on the comparability and validity of analytical data. The following principles that need to be considered in the reporting of quantitative test results are as follows:
● Form of the chemical hazard and unit of measurement: A test result should be reported in the same form (active – chemical form) and with the same unit of measurement as that given in the specified requirements (e.g., local regulatory limit, Codex Alimentarius).
● Number of significant figures: If the requirement provides clear guidance, the same number of significant figures should be reported. Otherwise, the test result should be expressed with one significant figure more than the limit stated in the requirement. In addition, the number of significant figures depends on the uncertainty of the analytical method.
● Correction for recovery: Generally, test results are not corrected for recovery. They may be corrected if the relative recovery is significantly different from 100% (typically <70% with good precision). In the latter case, both the measured and corrected value should be given, as well as the basis for correction. The recovery of a specific chemical hazard may vary, depending on the sample matrix.
● Reporting limits: Reporting limits are the LoD, which is key for banned or unauthorized chemical compounds, and the LoQ. As for the recovery, the LoD and LoQ of a specific analytical test method may vary depending on the sample matrix.
● Uncertainty of measurement: In accordance with the standard ISO/IEC 17025:2005, a statement on the estimated measurement of uncertainty (MU) should be included in test reports when:
● it is relevant to the validity or application of the test results;
● a customer’s instructions so require; or
● the uncertainty affects compliance with a specification limit.
● The final uncertainty is expressed as the interval (measured value ± expanded MU), at a
95% confidence level.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
● Uncertainty factors: However, some factors may contribute to discrepancies in the analytical results, and should be considered in further investigation in case of a non-compliance:
● Heterogeneity of the product batch/lot.
● Different sampling procedures for analytical testing.
● Different analytical testing procedures (including sample preparation, analytical method, quantification procedure, quality controls) with different performance characteristics (e.g., detection limit, measurement of uncertainty).
● Different “rules” to assess the regulatory compliance (e.g., correction for recovery, taking into account the measurement of uncertainty).
It is important to understand the principles that regulatory authorities apply to interpret analytical test results and how they assess the compliance of a product against a requirement. The application of different principles for treating data may affect the conclusion regarding the compliance or non-compliance of a product with a requirement. Many food business operators make use of external laboratories, or rely on the laboratories operated by suppliers or co-manufacturers. Governments also have their own control laboratories and may verify the compliance of products independently. In order to be able to rely on the results of tests, it is best to refer to ISO accredited laboratories.
APPLICATION OF THE HACCP SYSTEM TO MANAGEMENT OF CHEMICALS
Identification of Hazards
A first step in the management of chemical contaminants consists in identifying potential hazards associated with the product and manufacturing process. The source of many chemical hazards is the raw commodity or packaging material itself. Most chemical hazards present at source, i.e. raw material, will not be eliminated through processing. Some chemical hazards may also be formed during processing or storage. To identify potential chemical hazards, expertise is needed; hence the importance of integrating an expert on the subject into the HACCP team. As a complement, or in absence of an expert, the following sources of information can be consulted.
● Regulatory requirements (considering the requirements of the country where the product is to be sold). As products need to comply with the regulatory requirements, the contaminants that should be examined are those for which regulatory authorities have established some guidance or regulatory requirements.
● Scientific literature can provide information on the type of hazards which are associated with food, and their level of occurrence.
● Governmental and industry associations guidance material such as fact sheets, websites. The guidance provided by the International Life Science Institute (ILSI) or the Global Harmonization Database can be a source of such information.
● Reports of surveillance of governments or industry, be it monitoring of chemical contaminants in food and environment or reports of inspection of food control capabilities showing potential weaknesses in control, monitoring or analytical capabilities. A major food recall that occurred in 2001 in Europe in relation to
chloramphenicol in honey could perhaps have been anticipated if the report of EU inspectors, showing lack of monitoring and of governmental laboratory capabilities in China for enforcing legislation on veterinary drugs, had been shared with industry associations.
● Portals such as the RASFF (Rapid Alert System for Food and Feed). The accessible RASFF portal database at http://ec.europa.eu//rasff enables a search for RASFF notifications on food and feed of interest or a particular hazard. Certain chemical hazards, processing and packaging contaminants in particular, are best addressed in the design of the product. Therefore, their prevention and control must be considered during the early stage of product development and reflected in a preliminary hazard analysis using, e.g., the “Safety by Design” approach.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
Analysis of the Hazards
For hazards that are identified, a decision should be taken on their degree of risk. Those which are viewed as high risk in the HACCP study are referred to as a “significant hazard.” To identify which chemical hazard is significant, the following factors need to be considered:
● The likelihood of occurrence of the hazard above safety/regulatory limits. This may be estimated taking into consideration the factors influencing the occurrence of a hazard (see section on factors influencing the occurrence of hazards above) and the prerequisite programs in place. Data confirming the proper implementation of prerequisite programs must be available. Examples are audit reports of the supplier or manufacturing site and historical records such as monitoring data of the supplier.
● The severity of health consequences of the agent, taking into consideration the target consumer, the nature and the level of the chemical potentially present. If a regulatory limit or an industry limit is not available, the decision on the significance of a hazard could be based on food safety assessment. To this end, two types of data are required:
● A reference dose: this is the dose below which exposure to that chemical can be considered as safe (e.g., ADI, TDI, PTWI).
● An estimate of exposure3 based on food consumption data. The degree of significance of a hazard can then be estimated by comparing the level of exposure to the particular chemical agent through a given food with the ADI or other equivalent reference dose (TDI, PTWI). If the exposure does not represent a significant proportion of the safe reference dose, the agent is not viewed as a significant food safety concern (e.g., ratio of estimated intake to TDI or ADI <1). In other words, the significance of the hazard
can be evaluated based on the degree of contribution that it makes to the total exposure of the target consumer. In case that degree is negligible, the hazard is considered not to be a major food safety concern.
To calculate the level of exposure, the worst-case scenario must be considered, i.e. using the maximum consumption of the product and the maximum amount of chemical that may occur in the particular food, and based on historical records or other surveys.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
Control of Hazards
Except for hazards that may occur as a result of processing or storage, for a great proportion of chemical hazards, the control measures are at the supplier level, i.e., the application of GAP, GAH, GMP.4 For packaging contaminants, the design and formulation of the material as well as the application of specific GMP measures at the supplier level are the control measures. Thus, sourcing the raw material from reliable and approved suppliers is essential for preventing these types of chemical hazards. Therefore, the customer of a raw material must clearly communicate its requirements (including the intended use of the raw material) to the suppliers. Purchasing specifications is an important tool for this communication.
Testing the raw materials at reception is in principle a verification activity since it confirms the suppliers’ quality assurance program and compliance with the agreed specifications. However, in situations where the confidence level is low, it can be considered as a control measure, provided that it is carried out systematically on all lots of incoming materials, using a validated sampling plan. Results of the analysis will then be part of the release procedure. For some hazards, selecting resistant varieties of raw materials can be considered as one method to control a hazard, in which case the specific variety desired must be mentioned in the raw material purchasing specification. For processing contaminants such as acrylamide, the design and control of process parameters or the formulation of the product may constitute the main control measure. For certain types of mycotoxins, the control of storage conditions (storage time, temperature, humidity) of raw materials is the key control measure. For lubricants, food grade quality and good maintenance practices must be considered as key control measures (this is often done as part of GMP). Preventing accidental or cross-contamination with chemicals requires good warehouse
management, e.g., separation of cleaning chemicals from food items, proper closing and
labeling of chemicals, dedicated recipients, etc. The control of hazards must at all times ensure that chemical hazards are prevented, eliminated or reduced to an acceptable level.
Critical Limits
The second principle of HACCP is the decision on the critical limit. This is the limit which separates the acceptability from the unacceptability of a control parameter. These limits have to be established based on the parameters that characterize a control measure. For instance, if for the application of antibiotics, the control measure has to take account of a withdrawal period, the monitoring parameter is time, and the critical limit is the number of days required for the residues of antibiotics to decrease to an acceptable level, e.g., 7 days. However, for a raw material where there is low supplier confidence or the supplier is not known, and the testing of the raw material is considered as a means for controlling the hazards, the critical limit is the regulatory standard of the country where the raw material is to be used, or preferably the Codex norms if these are more stringent. For intermarket supplies, attention must also be paid to ensuring that the finished product meets the regulatory limits of the market where the product is sold and/or the Codex norm. If this requires a more stringent norm for the raw material than the regulatory requirement of the country where the product is manufactured, then this should be stated in the requirements communicated to the supplier. Chemicals used by producers (e.g., agrochemicals) or by food manufacturers (e.g., food additives) should not be used in food production and manufacturing if they have not been evaluated and have not been proven safe for use. For unapproved or prohibited chemicals, some governments may apply the concept of zero tolerance. This concept is based on the idea that if an agent is prohibited, its mere presence at any level is an indication of violation of the legislation. However, many in the product was inadvertent, a due diligence measure was taken to prevent it, the contaminant can have other sources, e.g., environment as was the case with melamine, the level in the product is so minute that it does not present a harm to consumers and corrective measures are taken to prevent it in future. Under such circumstances the governments may allow the product on the market. In other cases, the governments may confiscate the food and punish the producer, but destruction of such a food considered safe would not be wise. At very low levels of contamination, there may be conflicts among the stakeholders of the presence or absence of such a substance and regulatory authorities may determine minimum required performance limits (MRPLs) of the analytical method for such substances. For processing contaminants, the critical limits correspond to the acceptable limits of the processing parameter(s), e.g., temperature of the heat treatment. Similarly, for contaminants associated with storage, the critical limits will be the acceptable limits of storage parameters (temperature and/or humidity).
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
CCP Monitoring
Where the raw material is considered as a CCP, the chemical hazard must be tested on every batch and the results of testing must be made a release criterion. Correct and valid sampling is essential. Where the distribution of the hazard is heterogeneous (e.g., mycotoxins) and the raw material is considered as a CCP, the validation of the sampling method is particularly critical for food safety and must be considered as compulsory. Similarly, in line with HACCP principles, any processing or storage step which is identified as a CCP must be monitored; the parameters and frequency of monitoring must be set so that if the critical limit is violated, corrective actions can be applied in a timely manner. For chemical agents, re-processing is generally not applicable and an infringement of the acceptable level of the agent should lead to the rejection of the raw material or product. Personnel entrusted with the management of CCPs must be well trained, be aware of their responsibility and must completely understand the consequences of an eventual failure of the CCP.
CP Monitoring and Other Verification
In addition to CCP monitoring mentioned above, depending on the level of risk, a verification procedure must be established to confirm that control measures (preventive measures) are adequately implemented and the HACCP system is effective. For chemical hazards, verification includes activities such as:
● Audit of the supplier.
● Factory audit.
● Verification of identity of the raw material upon receipt in the factory (e.g., visual inspection) to confirm that the right variety is selected.
● Monitoring of the raw material for potential hazards according to the degree of risk as well as other factors (see “Monitoring Plans (see next section page 931),”).
● Testing of the finished products.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
Monitoring Plans
Frequency of Monitoring
Whether monitoring is implemented at a CCP for controlling a hazard or as a verification measure to verify that the control measures are applied correctly, the frequency of monitoring needs to be decided. In line with what has been mentioned above, this should be decided on a risk-based approach. However, frequently health risk is not a sufficient criterion since a one-time non-compliance may not present a significant health risk for the consumer or may even present no risk, but may jeopardize the reputation of the business or present economic risk in case of violation of regulations and product recall. Therefore,
a two-step decision-making process is proposed here. In a first step, the frequency of monitoring is decided taking into account:
● The likelihood of occurrence of the contaminant above acceptable levels in the raw material, e.g., taking into consideration the prerequisite conditions (e.g., availability of certificate of analysis).
● Health consequences for target consumers in case of non-compliance and the anticipated level of the contaminants in the final product.
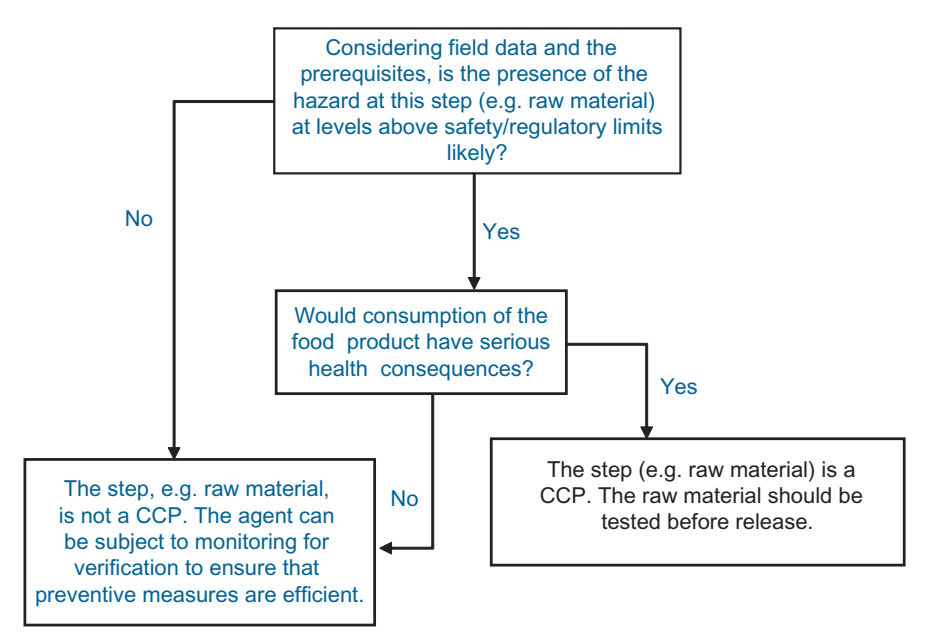
FIGURE .1 Simplified decision tree for hazard analysis of chemicals.
In line with the decision tree (Figure .1), depending on the level of risk, the raw material is considered as a CCP or Control Point (CP)5. If the risk is viewed as negligible, it may also be decided that no monitoring is required for food safety reasons. The monitoring frequency decided at this step should in principle set the minimum requirement for testing (Figure .2).
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
In a second step, the frequency of monitoring can be readjusted taking into consideration
“other factors and requirements”; examples are:
● Feasibility (availability of analytical method, speed of results).
● Regulatory requirements and/or public perception.
● Economic reasons: amount of raw material and/or product processes and implications in
case of a product recall.
● Impact on the image and reputation.
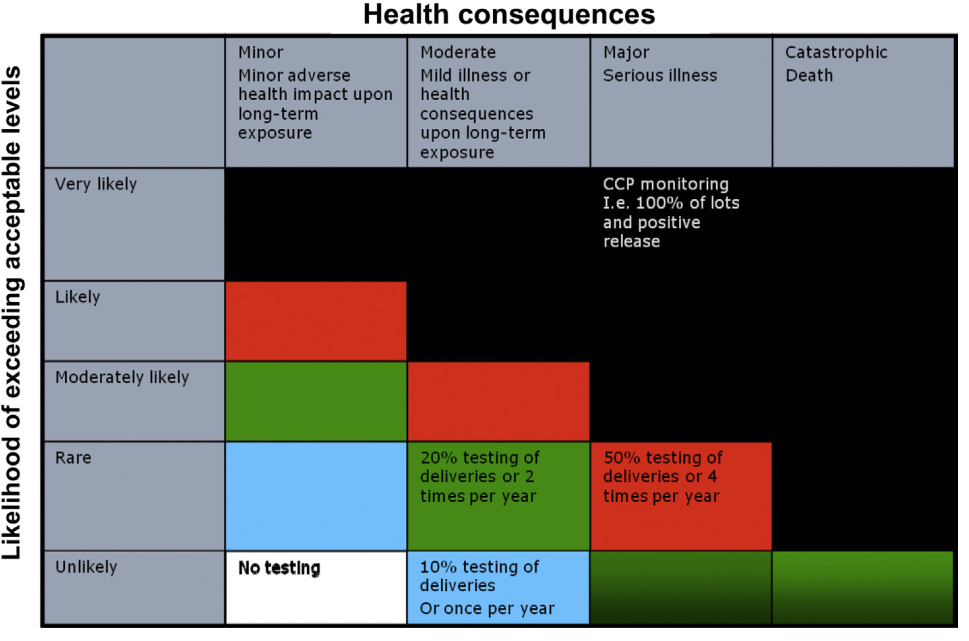
FIGURE .2 A schematic presentation of risk-based monitoring
Principles in Setting a Monitoring Plan
In setting up the monitoring plan, the following principles must be considered:
● Time of testing must be adapted to the period associated with the highest risk of contamination (e.g., monitoring of aflatoxin in cereals or nitrate in vegetables may require higher frequency in specific seasons or climatic conditions).
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
● Samples must be taken at the arrival of the raw material.
● A certificate of analysis can be required in place of, or in combination with, verification monitoring. However, the certificate of analysis must be obtained from an accredited or approved laboratory and be periodically verified internally.
● Except for contaminants that occur during processing and manufacturing, most of the efforts must be on the raw material. However, periodic testing of the finished products is also advised in the following cases:
● To verify that the HACCP plan is effectively implemented. This is important for significant hazards.
● To confirm compliance with the legislation.
● To verify that risks associated with processing or storage are under control.
● If a certificate of analysis is required by customers.
● If a certificate of analysis is required for exportation.
Corrective Action
In case of non-compliance, the following actions need to be considered:
● Non-complying raw materials must be rejected and suppliers must be advised. Depending on the situation and the gravity of the non-compliance, the need for increasing the frequency of the verification activities (i.e., frequency of auditing and/or monitoring materials for the potential hazards) or alternatively terminating the contract must be considered. Fraudulent practices must lead to an immediate termination of the contract.
● Non-complying finished products must be blocked.
● Any deviation must be immediately investigated and followed up.
● For chemical hazards occurring as a result of processing or storage conditions, a deviation from set standards must lead to a product reformulation or a change in processing or storage conditions.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
Validation
The principles of the HACCP system as well as of the Codex Alimentarius Commission require validation of control measures. Validation consists in obtaining evidence that the elements of the HACCP system are effective. As such, all the decisions relating to the different principles of HACCP need to be validated to ensure that they have a scientific and/or technical basis, and/or are based on accepted practices. These include consideration of the need for validating:
● Hazards which are considered as non-significant and efficiency of control measures (operational prerequisites), e.g., suppliers’ practices, monitoring and/or CoA.
● Limits and/or specifications.
● The sampling scheme and procedures.
● The analytical method (e.g., equipment, variability, sensitivity, approved and recognized
method).
● Frequency of monitoring activities.
● Training and competence of personnel, from operators to laboratory (e.g., accreditation).
Necessary data, records and documentation providing the basis for decision-making must be available.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
Maintenance of the HACCP Plan
Results of monitoring and other verification activities (e.g., audit of supplier, preventive maintenance) as well as previous records of consumer complaints and or accidents must be the subject of a continuous review. Hazard analysis and decisions on CCPs and/or frequency of monitoring and other verification activities must be re-evaluated and the HACCP and monitoring plan must be updated in the light of these data. Examples of technical or scientific data that should prompt an update of the plan are:
● Alerts (internal or external).
● Surveys by authorities or national food institutes.
● Reports or data on previous incidents or non-compliance.
● New scientific developments (e.g., emergence of new potential hazards).
● New or change in the regulatory requirements.
● Change of supplier or suppliers’ practices.
● Change in the country where the product is marketed.
● Change in the intended use, preparation method or target consumer.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
● Change in the product formulation or process/storage conditions; change in factors influencing the occurrence of a hazard, such as environmental contamination or climatic changes. The latter may for instance increase the risk of mold growth and formation of mycotoxins, which in turn can lead to abuse of fungicides. Climatic changes may also increase animal infections, leading to a higher use of antibiotics.
While the maintenance of the HACCP plan must be a continuous practice, it is a good practice to periodically review collected data and their trend analysis. Types of data that should be considered during such a review are:
● Results of in-house monitoring, including out-of-norm results.
● Survey or monitoring carried out by authorities (or planned to be carried out) or third parties.
● Verification of certificates of analysis.
● Performance of suppliers (audit reports, supplier’s monitoring plan) and future audit plans.
● Information on emerging chemicals.
● Reports on laboratory competences.
The results of this review must lead to an analysis of trends and decisions for:
● Enhancing preventive measures.
● Readjusting the frequency of monitoring.
● Setting up new monitoring activities or surveys.
● Communication to regulatory authorities regarding the feasibility of the legislation.
● Management of suppliers (request for audits, change in the frequency of monitoring or
issuing warnings).
In case of any report by regulatory authorities or other third parties (e.g., customer, consumer organization) of non-complying products, a transparent and speedy reaction is important to maintain credibility and the confidence of authorities. The following action is recommended: Handling of non-compliance and corrective actions – all non-compliances must be the subject of immediate follow-up action and must lead to the adjustment of the food safety management system, including monitoring activities.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
References:
Motarjemi, Y., Stadler, R., Studer, A., Damiano, V., 2009. Application of the HACCP approach for the management
of processing contaminants. In: Stadler, R.H., Lineback, D. (Eds.), Process Induced Food Toxicants and Health
Risks. John Wiley & Sons Inc., New Jersey.
Moy, G., Todd, E. Overview of foodborne illness and public heath aspects: Part 2. Chemical, physical and other
hazards. Encyclopedia of Food Safety. Elsevier (in press).
