INTRODUCTION
Microbiological testing programs play an important role in the verification of the effectiveness of control measures for many food products. Such programs may include monitoring1 of the production environment and processing equipment, and testing of raw materials,2 in-process and finished products. The relevance and application of testing programs depend upon the design of the product and process, the hygienic status of the processing environment and the availability of other verification information about a product lot. Microorganisms are often distributed unevenly in foodstuffs and the practicality and economics of sampling make product testing ineffective as control measures. Likewise, environmental monitoring provides a snapshot of the hygienic status of the environment or processing equipment at the time and locations that samples were taken. Product and environmental testing are lagging indicators of hygienic failures as they do not directly control the root conditions that lead to contamination. As such, they are most effective when used within a system of risk-based preventive controls, such as HACCP, hygienic zoning and other prerequisite programs, and when they work together with other verification activities to assess the condition of the food safety system. Microbiological testing may also be used to support the design and validation of control measures in a food safety management system. Testing may be used to determine initial microbial levels on raw materials or in-process product prior to the application of a microbiocidal process in order to establish the level of reduction required. Testing may also be conducted to determine the surviving levels of target microorganisms in a foodstuff after a microbiocidal process is applied in order to confirm that the desired reductions are achieved. It is often difficult to obtain quantitative information on the levels of pathogens present in a foodstuff prior to processing as levels of these organisms are often low and unevenly distributed, and information in verification testing programs and other surveys are based on analysis of presence or absence of the target organism, providing little information on population levels (ILSI Europe, 2010; Longaberger et al., 2012a,b). Where quantitative data are available for indicator organisms, it may be possible to extrapolate these data to estimate worst-case initial loads of the target pathogen. Most often validation studies are conducted using samples artificially inoculated with levels of target organisms sufficient to determine the reduction achievable by the process. Strains used in such studies are representative of those of concern in the foodstuff and are pre-conditioned to most closely approximate their physiological state prior to processing. Regulatory and industry reviews provide additional guidance on the use and application of microbiological testing in validation (ICMSF, 2011; NACMCF, 2006, 2010; Codex Alimentarius Commission, 2008b; Swanson et al., 2000; Zwietering et al., 2010).The remainder of this article will focus on the use of microbiological testing as verification in food safety management systems. The role of environmental, raw material and finished product monitoring programs will be discussed as well as approaches to their development and implementation.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
WHEN ARE MICROBIOLOGICAL TESTING PROGRAMS USEFUL FOR VERIFICATION?
Due to their cost and complexity, microbiological testing programs are only applied when they can provide relevant information about a product and process. Understanding the appropriate application of microbiological testing requires an understanding of the significance of microbial levels at various points in the process. The contribution of product, process and environmental factors to the ability to achieve the required microbiological limits in manufactured products is described in a conceptual equation developed by the International Commission on Microbiological Specifications for Foods (ICMSF). This equation illustrates the impact of various factors on the ability to manufacture product that does not exceed a food safety objective (FSO) or performance objective (PO; Codex Alimentarius Commission, 2007b, 2008b; ICMSF, 2002; Stringer, 2004; Van Schothorst, 2009; Motarjemi
and Moy, in press):
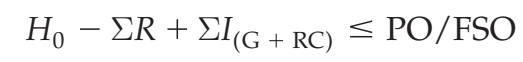
An FSO is the maximum frequency and/or concentration of a microbiological hazard in the foodstuff at the time of consumption necessary to achieve a public health objective such as an appropriate level of protection (ALOP). This is established by regulatory authorities as part of their risk management activities. Regulators may also define a PO, i.e. the maximum frequency and/or concentration of a hazard in a food at a specified step in the food chain
before the time of consumption, in order to meet an FSO (Figure 1). Likewise a performance criterion (PC) may be established to communicate the required outcome for a control measure or series of control measures, such as microbiocidal or micro biostatic controls (Codex Alimentarius Commission, 2007b, 2008b; ICMSF, 2002, 2011). A PO or PC may also be developed by the manufacturer based upon an established FSO, where one exists, or based upon the levels of relevant microbiological hazards necessary for product safety as determined in the HACCP study. The ability to produce a product that is equal to or below the PO is based upon the initial microbial levels in raw materials (H0), the
presence of conditions that could increase microbial levels during storage and processing (ΣIG), the presence of conditions that could lead to the recontamination of the product or raw materials from equipment or the factory environment (ΣIRC) and the microbial reduction achieved as a result of process controls (ΣR). Such an understanding of the contribution and interrelatedness of factors affecting microbiological quality and safety can assist those developing a product or process to determine the appropriate combination of control measures to ensure product safety. It can also be used to determine the application and nature of the corresponding monitoring programs necessary to verify the effectiveness of these controls. Examples of the application of microbiological monitoring programs for the verification of various products are provided in Table 1. Testing may be useful to verify the hygienic
status of raw materials that are not subject to a lethal process, either in a dry mix or assembly
operation, or where they are added after the application of a lethal process. Testing may also
be useful for raw materials that are exposed to conditions that would allow the outgrowth of
microorganisms to levels greater than that which the applied processes are capable of inactivating. Testing is generally unnecessary for raw materials that will be subjected to a process that will inactivate the levels of pathogens or spoilage organisms present in the raw materials.
Environmental monitoring may be necessary to verify the application of environmental controls where raw materials, in-process or finished products are exposed to the production environment without a subsequent microbiocidal step. Such monitoring may not be relevant for products that are enclosed during processing and packaging unless the hygienic condition of the environment where the finished product is handled may have an impact on the ingress of microorganisms (for example, contact with cooling water or poor hygienic handling of retorted products prior to cooling).
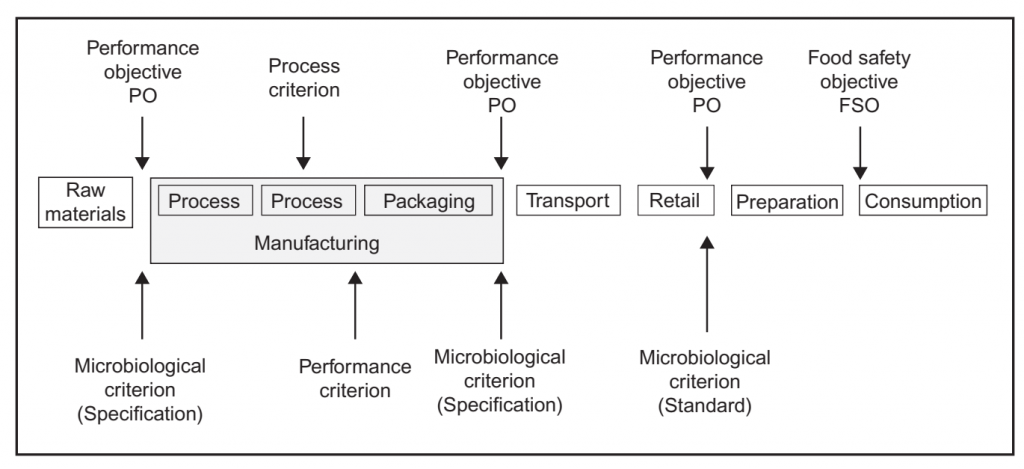
FIGURE 1 The role of food safety objectives, performance objectives and microbiological criteria in food
safety management (adapted from Gorris, 2005; Codex Alimentarius Commission, 2007b).
TABLE 1 Examples of Testing Applied to Products and Process Controls
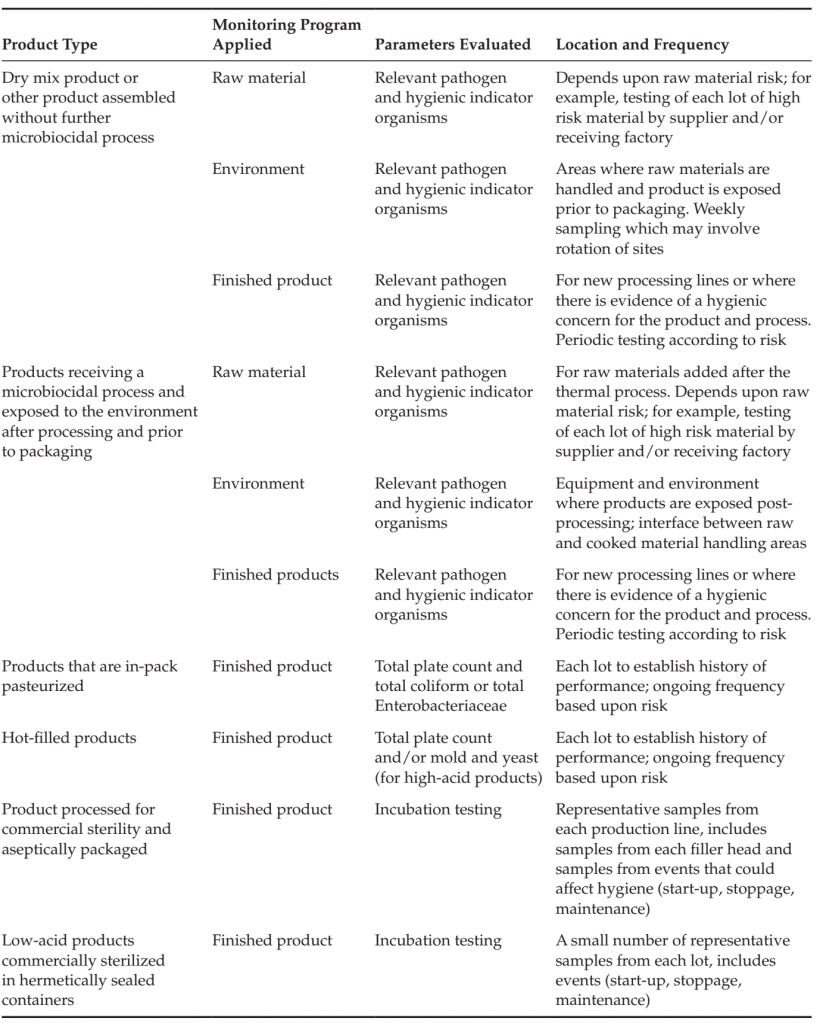
A finished product testing program may have a role in verifying the overall functioning of preventive control measures for products that rely on various supplier, production and environmental controls, such as products blended or assembled without a subsequent lethality control measure, or products exposed to the environment following the application of a microbiocidal process. A finished product testing program is less relevant for products that receive a process in the final package, but may play a role in verifying the application of a thermal process, evaluating the functioning of a production line over time or investigating potential process failures. Details on the application of raw material, environmental and finished product monitoring programs are discussed in subsequent sections of this article.
PREREQUISITES TO THE DEVELOPMENT AND IMPLEMENTATION OF MICROBIOLOGICAL TESTING PROGRAMS
Effective product testing and environmental monitoring programs are developed and implemented only after the implementation of programs that identify and establish appropriate preventive controls:
● Hazard analysis and critical control point system;
● Hygienic design of equipment and processing environment;
● Hygienic zoning controls to prevent entry, harborage and growth of pathogens;
● A well-designed raw material selection and verification program;
● Personnel training to ensure that control measures are applied correctly.
Microbiological testing is of limited value in the absence of such preventive controls;
however, microbiological testing and monitoring programs can be effective tools for verification when they are based upon a thorough understanding of the product and process as determined in these programs.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
Requirements of Regulatory Agencies and Customers
Finished product requirements may be defined in customer specifications or in regulatory requirements. Regulatory requirements may be expressed as FSOs at the point of consumption or as POs for the inished product after production or for product on the market. Often, requirements are expressed indirectly as within-lot microbiological criteria for lot acceptance, increasingly following the format developed by ICMSF and adopted by the Codex Alimentarius Commission (Figure 1). For some products, betweenlot criteria are established as an ongoing assessment of process control (ICMSF, 2002; Codex Alimentarius Commission, 1997). Some regulatory bodies have systematically established microbiological criteria for relevant categories of ready-to-eat products (e.g. Canada, European Union, Hong Kong). Other regulatory bodies have developed criteria for finished products or raw materials as needed based upon an identified risk or in response to the occurrence of public health incidents or a specific public health concern (NRC, 2003). Where such criteria exist they can be used to determine the appropriate design of products and process controls necessary to meet these criteria, and the testing programs necessary to ensure that the criteria are consistently met. Microbiological criteria should not be mistaken with FSO or PO. The former define the acceptability or unacceptability of products (in or out), the latter is used for designing the control measures, defining the expected/ desired performance in verification programs and establishing expectations in contractual agreements. In many cases there are no criteria specified for a product in regulation or in customer requirements; instead, there is a general requirement for the producer to manufacture safe products. It is therefore the producer’s responsibility to determine the necessary PO, PC, microbiological criteria and supporting verification programs for raw materials, processing environments and finished products. For some products, industry guidance has been developed to support manufacturers in the development of appropriate product and process criteria (Chen et al., 2009a, b; GMA, 2010; MAF/NZ, 2011; NFI/NFPA, 2002; Scott et al., 2009).
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
Hazard Analysis and Critical Control Point Study
Microorganisms of concern for the product and process are identified in the hazard analysis conducted as part of the HACCP study. The study will identify at what point in the process microorganisms will be introduced or multiply and will identify the type and location of control measures necessary to ensure the hazards are controlled. This will be based upon an understanding of the microbiology of raw materials, the effect of processes applied during manufacture, the exposure of the product or raw material during processing and after the application of a microbiocidal process, the behavior of the pathogen in the product (survival, growth, inactivation) and the impact of consumer preparation and reasonably expected misuse. The HACCP study will also identify the procedures, including microbiological monitoring, that are necessary to verify the ongoing functioning of the preventive controls for the identified hazards. The HACCP study is focused on microorganisms of food safety concern; however, information from the study can also be used to evaluate the impact of product attributes, handling and distribution conditions on product spoilage and thus the relevance of nonpathogenic spoilage organisms in testing programs.
Zoning of the Factory Environment and Hygienic Design of Equipment
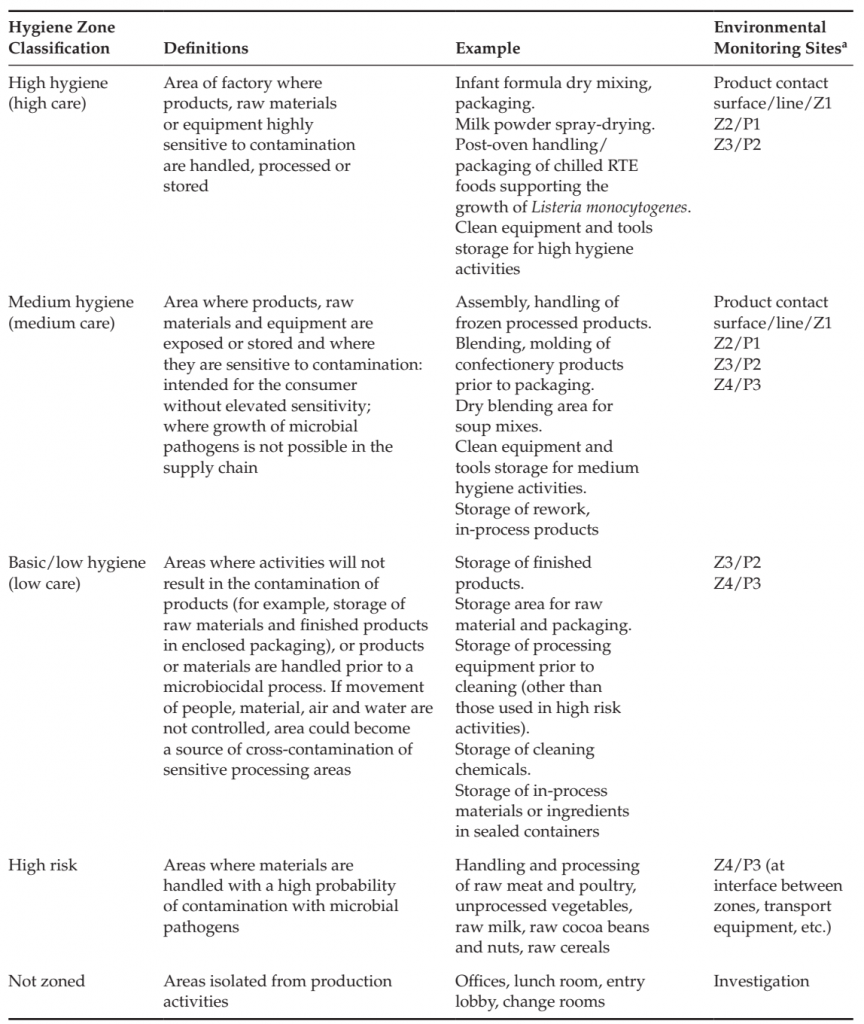
Hygienic zoning is the separation of factory areas based upon the risk of product contamination and the corresponding hygienic and preventive controls necessary to ensure that cross-contamination of products and raw materials does not occur (Duffy et al., 2003; Holah, 2005; Scott et al., 2009). Such controls may include physical barriers, cleaning practices, restrictions on the control of the movement of people, materials and equipment, management of tools, air flow and personnel practices required for each area and for movement between areas. Control measures include those specific to the area as well as those necessary at the entry to or transition between zones, for example between areas that must be dry cleaned and areas that are wet cleaned, or between areas where unprocessed, highly contaminated materials are handled and where products are handled that have received a microbiocidal process, or where raw materials are handled that will be used in a process without application of a microbiocidal process. Zoning studies consider product design, process flow, equipment design, exposure of raw materials and product before and after microbiocidal processes, movement of people, materials, equipment and waste, air and utilities flow, prior history of the product type and processing facility. The studies identify sensitive areas of the process (e.g., areas where product is exposed to the environment), high risk areas and activities (handling of highly contaminated material such as raw meat, maintenance activities, management of waste) and factors that could lead to cross-contamination into sensitive areas. Good hygienic practices, structural and logistical control measures are identified including cleaning and sanitation practices necessary to ensure protection of the product from contamination. Areas of the factory are classified according to the required hygienic controls (Table 2).
The most stringent controls may be needed at the interface between high-risk activities and areas of the factory where ingress of pathogens into processing areas can occur (through personnel or other activities), for example:
● To protect areas that must be kept dry to prevent harborage with Salmonella from other areas of the factory that must be wet cleaned;
● To ensure that pathogens present in materials where their presence is likely do not enter into production areas where product is exposed following a microbiocidal process;
● To ensure control of the environment where product is exposed that is intended for sensitive populations;
● To ensure the application of hygienic controls is sufficient to prevent the contamination of perishable chilled products with Listeria monocytogenes that may grow during storage of the product.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
Sampling sites at such interfaces will be included in environmental monitoring programs to evaluate the effectiveness of control measures. Specific examples of zoning controls are available in regulatory and industry guidance on the control of food borne pathogens (Chen et al., 2009a, Codex Alimentarius Commission, 2007a, 2008a; US FDA, 2008; USDA FSIS, 2012; GMA, 2010, 2012a; NFI/NFPA, 2002;
Tompkin et al., 1999). The outcome of zoning studies is often included in zoning maps, which identify the hygienic classification of areas, and includes the movement of people, materials, equipment, waste and air. Such maps are valuable in identifying relevant sites to be included in environmental monitoring programs. A study of the hygienic design of equipment and manufacturing environment will help to identify where potential harborage points exist in the process. These include points where food or water can collect and/or which are difficult to clean. In many cases the identification of such areas of concern in hygienic design and zoning studies, such as harborage points or hollow bodies in equipment or production areas or traffic patterns, will result in corrective actions to address the concern. Until these areas can be addressed, they will be under increased scrutiny in environmental monitoring programs.
TABLE 2 Hygienic zoning Classifications and sample Prioritization
MICROBIOLOGICAL MONITORING OF THE FACTORY ENVIRONMENT
Environmental monitoring programs are used as a verification of the effectiveness of control measures to prevent the ingress, harborage and multiplication of microbial pathogens in the production environment, specifically:
● Effectiveness of cleaning and sanitation procedures;
● Effectiveness of environmental controls:
● Controls associated with hygienic zoning
● Movement of people, equipment and materials
● Construction and maintenance activities
● Identification of areas of ingress or harborage so that they can be eliminated;
● Investigation of the impact of adverse findings.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
The application and design of environmental monitoring programs will depend upon the risks associated with the product and process. For example, an evaluation may not be needed of the processing areas for products that are processed in enclosed systems and filled aseptically or hot-filled. Likewise, products that are processed in their final package and are not exposed to the environment after processing may not require stringent sampling programs unless there is a risk of recontamination (for example, through micro-leaks in the seams of cans during cooling following a thermal process). While an environmental monitoring program can be a valuable verification tool, it only provides a picture of the sites analyzed during the day samples are taken. However, when evaluated along with other samples taken from a production line or process environment over time, it can provide useful information regarding ongoing status or trends in hygienic control.
Selection of Pathogens and Indicator Organisms
The pathogens that are the focus of the environmental monitoring programs will be determined by the hazard analysis in the HACCP and zoning studies. Generally, Salmonella and Listeria monocytogenes are the pathogens of environmental concern, although other pathogens may be included based upon product and risk (for example, Cronobacter spp. in infant formula; Staphylococcus aureus or Bacillus cereus as investigative sampling in areas where outgrowth of the organism during processing is a concern).
Environmental monitoring programs include the pathogen of concern; however, the infrequent or sporadic distribution of these organisms often makes them difficult to detect, even when they are present in the factory environment. A program focused solely on the isolation of the target pathogen will only identify a problem when it occurs and may not identify early enough that conditions are or have been present that would also allow the ingress or growth of the pathogen of concern. Because of this, effective environmental monitoring programs include hygienic indicators, selected according to their ability to demonstrate the presence of conditions that would lead to the presence or growth of the pathogen of concern.
Processing Environments where Wet Cleaning is Conducted
Listeria monocytogenes is the primary environmental pathogen of concern in processing environments where wet cleaning is used. The most effective indicator organisms for the presence of L. monocytogenes in the environment are other members of the Listeria genus (USDA FSIS, 2012; US FDA, 2008, 2013). Because they are very closely related to L. monocytogenes, the detection of non-monocytogenes members of the Listeria genus (Listeria spp.) indicates that conditions exist that could also lead to the presence of L. monocytogenes. Detection of these indicators will initiate a root cause analysis and increased investigative testing of equipment or the environment from which the isolation occurred to ensure that the root cause is investigated. Recovery of Listeria spp. or L. monocytogenes from product contact surfaces or nearby areas on equipment or the environment may also initiate or intensify finished product testing to verify that the product is not affected. Quantitative indicators, such as Enterobacteriaceae, coliforms or total plate counts, may be useful for monitoring the effectiveness of cleaning and sanitation procedures or to assess whether conditions exist that allow multiplication of microorganisms on or around processing equipment. Because they are heat sensitive, Enterobacteriaceae and coliforms are useful for the evaluation of the hygienic status of the processing line after a thermal process. Total plate counts (TPC) may be used to monitor those conditions are present during processing that could lead to the outgrowth of S. aureus or B. cereus. High TPC results are followed by investigative sampling of potential harborage sites in or on processing equipment or holding containers (e.g. tanks, totes, mixers), where product and moisture may be present that could lead to the growth of these organisms. Such testing is usually conducted as an investigation of out-of-specification results from finished products. Because TPC is a quantitative hygiene indicator, expected baseline levels are established through an analysis taken of clean surfaces when it is known that cleaning and sanitation were effective. Due to the broad variety of microorganisms that will be recovered for TPC analysis, it is most effectively used for the evaluation of product contact surfaces or nearby surfaces and is less useful for areas of the environment away from the processing line. The inclusion of mold and yeast may be useful in monitoring programs for the exposed product environment of products for which yeast and mold spoilage are a concern (such as chilled dairy products, intermediate moisture pasta, etc.). ATP bioluminescence involves the detection of adenosine tri-phosphate (ATP) present in food material through the generation of a luminescent signal expressed in relative light units (RLU). The intensity of the signal is proportional to the level of ATP present and is an indirect indicator of the amount of biomass present. ATP bioluminescence may be used to verify the effectiveness of cleaning by measuring the presence of signals originating from residues present on product contact surfaces after cleaning (Moore et al., 2001; Powell and Atwell, 1997; Whitehead and Smith, 2008). To properly evaluate signals detected by bioluminescence equipment a baseline signal is established through the analysis of clean surfaces. An elevated signal will indicate that product residues are present, indicating that cleaning was inadequate and the surface needs to be recleaned.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
Processing Environments that are Dry Cleaned or Controlled-wet Cleaned
Salmonella is the primary pathogen of concern in factory environments where dry materials are handled or low moisture products are manufactured and where dry cleaning, or in specific cases controlled-wet cleaning, is applied to ensure the absence of moisture from the environment during processing (Duffy et al., 2003). Salmonella can enter the environment through the movement of people, equipment and materials or through the failure of zoning controls between high risk and sensitive areas (for example, between areas where raw cocoa beans are stored and handled and roasted cocoa beans and cocoa products are exposed to the environment). When moisture is present, Salmonella can multiply; however, even where moisture is absent, the organism can persist for long periods up to years and multiply when moisture re-enters the environment (Scott et al., 2009). Resident strains may remain dormant only to reappear after some time due to a change in activity, such as a construction or maintenance event, or hygienic failure allowing the ingress of water. Unlike the Listeria genus, there is currently no microorganism identified whose presence will closely correlate with the presence of Salmonella. E. coli has been used as an indicator of fecal contamination in water and as an indicator of post-process contamination in dairy products. While the monitoring of E. coli in the environment may be part of a monitoring program where fecal cross-contamination or growth is suspected, E. coli can persist in the environment and its presence in dry environments may not correlate directly with the presence of Salmonella (Cox et al., 1988; Kornacki and Johnson, 2001). Salmonella is a member of the Enterobacteriaceae family and the quantitative analyses of the environment for members of this family are frequently included in pathogen monitoring programs for dry environments. Unlike Salmonella, which may only enter the environment rarely through a hygiene failure, many members of the Enterobacteriaceae family are likely to be present at some level even in clean environments. An evaluation of the level of Enterobacteriaceae present at a sampling site can provide information on whether conditions are or have been present that could lead to the multiplication of Salmonella. As Salmonella may or may not be present in the environment, there is not a direct correlation between the presence or population of Enterobacteriaceae and the presence of Salmonella; however, the use of quantitative determinations of Enterobacteriaceae in environmental monitoring programs will allow conditions that may lead to the multiplication of Salmonella to be identified and corrected. Enterobacteriaceae should only be included on product contact surfaces or near product contact surfaces due to the variability of levels in non-process areas of the factory without strict hygiene controls. (Figure 2) For products intended for infants, Cronobacter spp. is a significant concern and is included in environmental monitoring programs for infant formula manufacture where ingredients, in-process or finished product are exposed. As with Salmonella, Cronobacter spp. is a member of the Enterobacteriaceae family and control measures taken to prevent Salmonella entry and harborage in the environment will also be effective for this organism. Cronobacter spp. has greater prevalence in the environment than Salmonella, increasing the importance of proper management of control measures and the corresponding stringency of monitoring programs. As with Salmonella, inclusion of quantitative Enterobacteriaceae as a hygiene indicator can help to identify the presence of conditions that could lead to Cronobacter spp. harborage and growth (Codex Alimentarius Commission, 2008a).
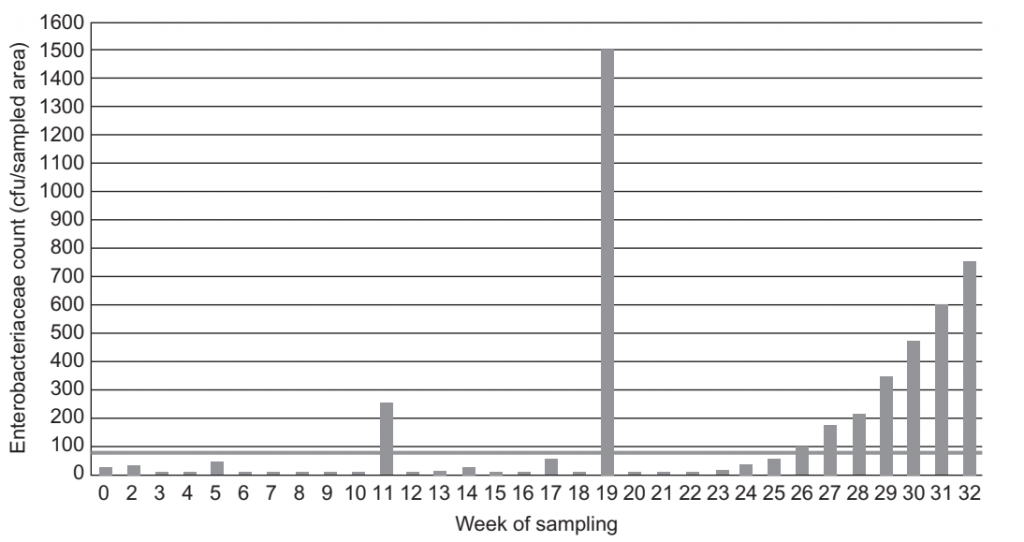
FIGURE 2 Enterobacteriaceae count at sample site A64 (near-product contact).
TABLE 3 Examples of Reaction Limits and Interpretation of Quantitative Enterobacteriaceae in Product
Residue Taken from Equipment surfaces and Process Environment in a Powdered Milk factory.
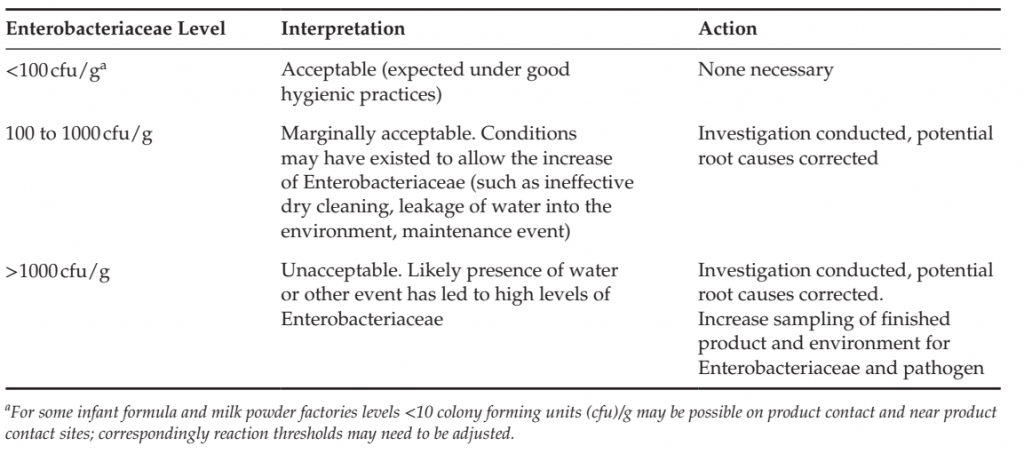
Enterobacteriaceae is a quantitative indicator, and baseline levels and reaction limits need to be determined to facilitate the interpretation of results and corresponding corrective actions. Levels should be established with an understanding of the product and process and what is achievable under good manufacturing practices. An example of such limits for a milk powder factory is provided in Table 3. ATP bioluminescence may be applied to verify the effectiveness of a periodic wet-cleaning process, but is typically not useful for environmental monitoring programs in factories manufacturing low moisture products that are dry cleaned as the presence of product particulates on equipment surfaces will interfere with the ATP signal.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
Selection of Environmental Monitoring Program Sites
Sample sites for environmental monitoring programs are selected based upon risk as identified in HACCP, zoning and hygienic design review, with the primary focus on the following areas:
● Areas of equipment that are difficult to clean and could be harborage sites;
● High-traffic areas;
● Interfaces where movement occurs between hygiene zones;
● Interfaces between areas were raw, highly contaminated materials are handled and where
processed materials are handled after the application of a microbiocidal process (such as
cooking);
● Interfaces between wet- and dry-clean areas;
● Areas from where pathogens could be transferred into sensitive areas with exposed
product or raw materials through the movement of people, equipment and materials;
● Areas where pathogens could enter into the facility from outside the factory or from
higher risk areas within the factory.
Sampling sites are classified according to the potential for product contamination if the pathogen was present at that site. Some companies have classified samples according to sampling “zones” while other companies have used other terminology for sample prioritization to avoid confusion with the classification of hygiene zones (Table 4). In fact, there may be a variety of sites of different risk classification within a given hygienic zone (Table 2). The location and number of sampling sites will vary based upon the nature of the product, the complexity of the process, the degree of product and raw material exposure, the GMP practices necessary for a particular production area, and the movement of people, equipment and materials. Examples of potential sources and harborage sites for Salmonella and L. monocytogenes in food processing facilities are available in guidance documents (Chen et al., 2009b; US FDA 2006) and can be valuable resources in establishing sites of focus for hygiene audits and environmental monitoring programs. The weight of sampling programs is placed on the most sensitive sampling locations. This is reflected in the number of samples selected and the sample frequency, with priority given to more sensitive areas. Product contact surfaces are analyzed according to risk of product exposure and sampling history. The number of sampling sites that are included in an environmental monitoring program is based upon the nature of the product and process and the design of the processing line and factory. The number of samples taken on a given sampling day will often be weighted based upon sensitivity (for example, a proportional split of P1/Z2 60%, P2/Z2 30%, P3/Z3 10%). Greater emphasis will also be placed on historically problematic areas, or those where an investigation has identified conditions that may lead to ingress or harborage.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
TABLE 4 Prioritization of sampling sites
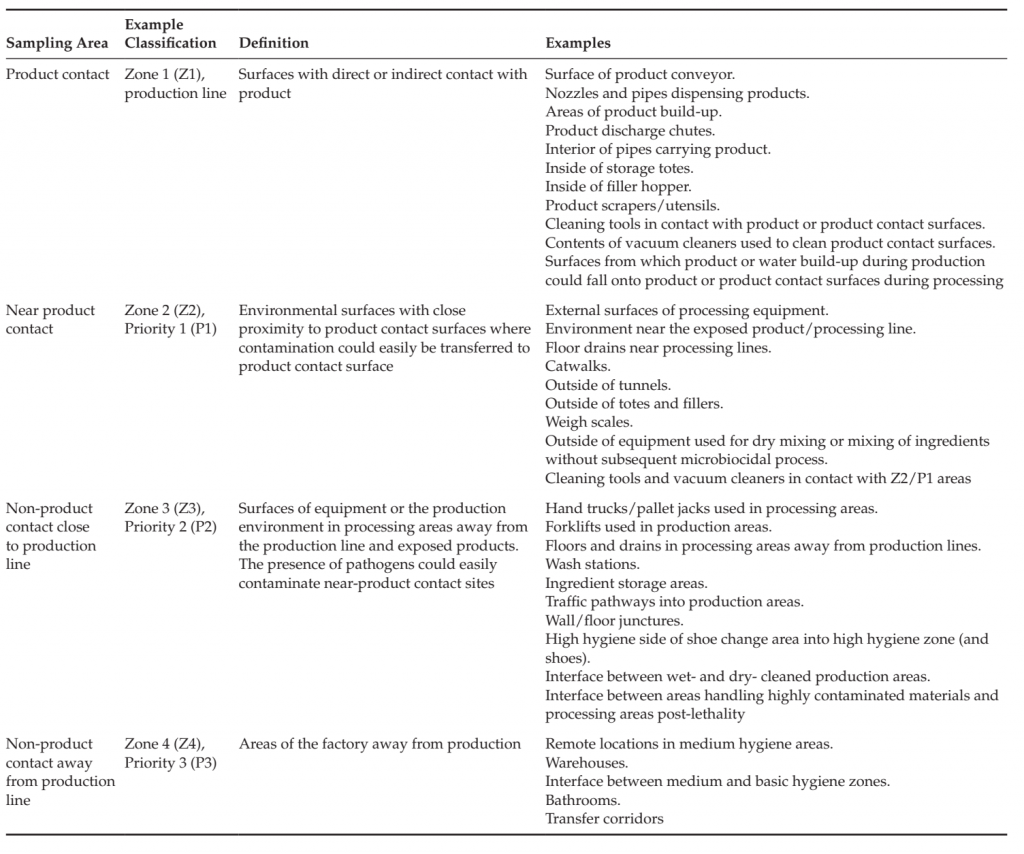
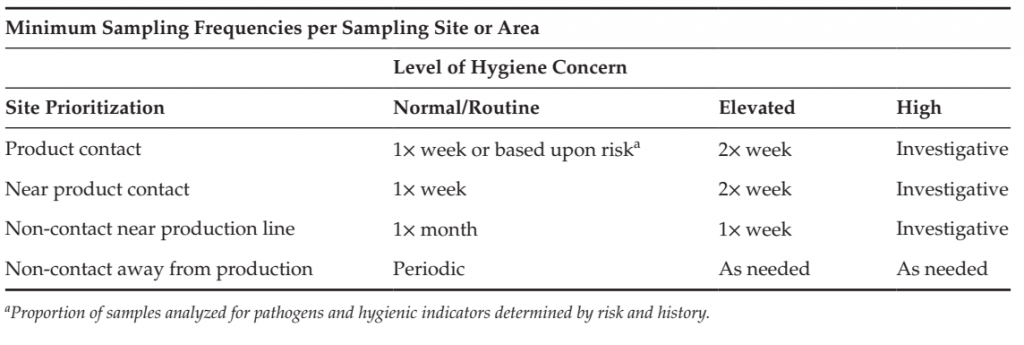
TABLE 5 Example of sample frequency based Upon site Prioritization and Level of Hygiene Concern
An example of sample frequency is included in Table 5. The frequency of sampling will vary based upon risk; however, sampling in most cases is conducted weekly and increased as a result of a finding (event potentially affecting hygiene, hygiene inspection finding, finding of pathogen or adverse trend of hygiene indicator in the environment). In some cases, sampling will be conducted less frequently, for example according to a production schedule where a product or processing line is only used infrequently. “Due diligence” programs that only involve infrequent sampling occasions (such as monthly, quarterly or bi-annually) are generally not useful as they provide little information of the hygienic status of a process and do not allow a rapid correction of hygienic failures and adjustment of sampling programs when adverse results are obtained. As their selection is based upon a risk assessment, the majority of sample sites in an environmental monitoring program are predetermined. Sampling programs should include a proportion of investigative samples, taken based upon the results of hygiene audits or of observations taken at the time of sampling. Established sampling sites may be modified based upon monitoring program findings. Where a number of sampling areas are identified, sites may be rotated with a given number of sites sampled at each sampling occasion. For example, a factory may select samples randomly using a numbering system classified by sample priority. Some factories have assigned sampling sites to alternating sample schedules, for example 1 week sampling is conducted for 50% of sites according to Schedule A, the subsequent week for the other 50% of sites according to Schedule B. In most cases the
same sites are evaluated for hygiene indicators and for pathogens. Sites are ideally identified on detailed factory maps, which may include information on hygienic zoning of areas and movement of people, equipment and materials. This facilitates the interpretation of data and also allows communication of data to factory personnel, or to corporate microbiologists or sanitarians in a different location supporting the factory in troubleshooting problems. The stringency of an environmental monitoring program should be adaptable, increasing upon adverse findings, events or insufficient information on hygienic conditions. The increased intensity is reflected in more frequent sampling, but also may be reflected in an increased number of sampling sites and investigative sampling focused around the area of the adverse finding.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
An example of program adaptation is included in Table 5. In this example, sample frequencies are categorized by normal/routine, elevated and high concern. The elevation of sample frequency may be applied to a specific production line where it is isolated from other lines, or to a specific processing area that is the focus of the hygienic concern. Elevated concern could result from:
● Elevated level or adverse trend in quantitative hygiene indicator;
● Maintenance event;
● Exposure of factory area to the adverse conditions potentially impacting hygiene (for example, roof leak, sprinkler operation, burst pipe);
● Breach of hygienic zoning controls;
● Finding of pathogen in the processing area away from processing line. High concern could result from:
● Finding of pathogen or out-of-specification hygiene indicator (presence of Listeria spp., elevated Enterobacteriaceae) in product, on product contact surface or the environment near the processing line.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
The increased program intensity continues for a time sufficient to verify that the hygienic status of the line has returned to normal. This could vary due to the nature of the problem leading to elevated concern and the sensitivity of the product. For example, increased sampling may only be needed for a short period following a maintenance event. Sampling at elevated or high concern may be continued for a longer period of time where evidence (hygiene audits, test results) indicates a persistent problem or where investigation of a positive pathogen finding has not determined a clear root cause. Likewise heightened sampling may be conducted for several weeks or months for a new factory or production line. A line
sampled under a high concern level may be placed on a sampling program for elevated concern for a period of time before returning to routine sampling.
Collection of Environmental Samples
As important as the selection of sampling locations to the success of an environmental monitoring program is the effectiveness of sample collection procedures. Samples collected immediately after cleaning and sanitation will verify the effectiveness of this operation and the suitability of the line for the start-up of production. Samples collected during production may indirectly verify cleaning effectiveness but will also verify the effectiveness of control measures aimed at preventing contamination of processing areas or production lines, harborage or growth. Samples collected at the end of production will verify control measures but may provide additional information on microbial growth during production and can provide information on the risks associated with production, build-up of material on the line and the intervals between cleaning and sanitation activities. Samples taken towards the end of a production run are recommended; however, some sampling programs include a combination of samples taken post-sanitation and samples taken during production. The tools selected for sampling will depend upon the nature of the site to be sampled as well as the level of residue/debris present at the site. Sterile, pre-moistened swabs may be most effective for the sampling of small cracks and crevices on equipment and the environment where moisture or product may collect, and for difficult-to-access areas. Sterile, premoistened sponges are more effective for sampling larger sampling areas on equipment and the environment. Sterile spatulas or scrapers may be used to sample product residue. Other tools, such as sterilized disposable dusting cloths or mop heads, may be useful tools for collecting samples from large areas in the environment during root cause investigations. Pre-moistened swabs and sponges are available from several manufacturers, in some cases with novel features that facilitate sampling of difficult areas and aseptic transfer of
sponge to neutralizing buffer or other appropriate transport medium. In cases where sponges are pre-moistened prior to use, it is important to squeeze the majority of moisture from the sponge prior to sampling. Where pre-moistened swabs or sponges are used in processing areas or processing equipment that must be dry during production, the sampled areas are dried after sampling by the technician taking the sample. Sampling with swabs and sponges should use sufficient force to ensure that any contamination present in the sampled area is transferred to the sponge. In many cases, defined areas are sampled (e.g. 50cm2) which could be identified using a sterilized template, to facilitate comparison of quantitative results between sampling sites or trends in the same sampling site over time. Agar contact plates are sometimes used to sample equipment surfaces. Sampling is conducted through direct contact with the surface being sampled. Plates are then covered and incubated until colonies develop which are then enumerated. The advantage of such methods for the analysis of quantitative indicators is that they require little or no advanced preparation and no additional preparation after sampling other than incubation. However, they are limited in their ability to transfer contamination present in cracks and crevices in equipment and may lose effectiveness on surfaces with a large build-up of soil. Where present, the sampling of product residue or soil is preferable to the sampling of “clean surfaces” as such residue is more likely to be a source of harborage. Samples are taken with a sterilized brush, spoon, scraper or spatula, depending upon the material collected, and transferred to a whirl-pack bag. When sampling build-up of product on surfaces
or the environment, care should be taken not to focus on the sampling of clean product, but
instead to focus activities on areas where the build-up of product and/or moisture could
lead to microbial harborage and growth. Samples should be transported in a suitable buffer or other transport medium. In cases where the residuals of sanitation chemicals may be present, in particular when sampling following a cleaning/sanitation event, a neutralizing buffer should be used. If not analyzed immediately after collection, samples must be stored under refrigeration (0–4°C) until they are analyzed. (In some cases, dry samples may be stored at room temperature if storage does not affect the survival or level of the target organism or group.) If analyzed off-site, samples must be shipped under refrigeration, with care taken to ensure that the refrigerant (such as an ice pack)
does not freeze the sample. Samples need to be analyzed soon after they are taken, preferably within 36 hours (Andrews and Hammak, 2003; Evancho et al. 2002; Midura and Bryant, 2001).
In some cases, for example when sampling specific high priority sites, the same site is analyzed at each sampling event. However, many locations identified for sampling will be areas of the equipment or factory environment; for these areas specific sampling sites are varied at each sampling event during routine sampling. When separate samples are collected from the same site, for example for Salmonella and total Enterobacteriaceae, care must be taken not to swab the same area for both samples at the same time; in these cases, adjacent areas are sampled for the hygiene indicator or pathogen. Investigative sampling will be conducted in the event of a pathogen finding or out-of specification hygiene indicator, or will be conducted when issues are observed during sampling or during a hygiene audit that could impact safety. Following a pathogen finding, sampling typically involves re-examination of the location of the finding and the surrounding area and may also include strategic sampling of other areas of the factory to investigate the extent of contamination, the movement of the contaminant through the environment and/or the origin or harborage point of the contaminant. When investigating potential harborage sites in processing equipment, it may be necessary to shut down and open the processing line to allow access to sampling sites. In such cases sampling is done during a
scheduled or unscheduled shutdown, or a specific shutdown is scheduled to allow a sufficient examination of the equipment. This is particularly important in an investigation of a pathogen finding. During routine monitoring, samples are sometimes pooled (i.e. combined into one sample) for analysis. Such pooling is generally done across similar areas or sample prioritization sites (for example, samples taken from product contact surfaces) on a production line or in
a production area. Pooling is not recommended across production days, between production lines or between sites of different prioritization. The advantage of pooling is greater efficiency of cost and the ability to sample more sites in the program. The disadvantage is that the source of contamination is more difficult to trace when adverse results are found (USDA FSIS, 2012). For this reason, pooling is not recommended for sampling when conducted under elevated or high concern.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
Analysis and Interpretation of Environmental Monitoring Data
Monitoring data from a sampling event represents the hygienic status of processing equipment or the processing environment at the time that the samples were taken. For samples taken in response to an event (maintenance, observation of hygiene failure), such data could indicate the impact of the failure, or, if taken following corrective actions, indicate the effectiveness of those actions. The presence of a pathogen or out-of-specification hygiene indicator are lagging indicators of a failure of hygienic controls which has led to the presence or harborage of
pathogens, or to the presence of conditions that could potentially lead to the growth or multiplication of pathogens. Examples are elevated Enterobacteriaceae in dry environment, elevated coliform in product contact sample and Listeria spp. in “wet” processing environment. Unless observations of hygiene deviations were made at the time of sampling, additional investigation will be needed to determine the root cause of the failure and to ensure that any corrective actions taken were effective. Depending on the location of the out-of-specification sample, finished product sampling may be needed to verify that product was not affected. Where pathogens are isolated from product contact surfaces, it is assumed that corresponding product that has made contact with the surface is also positive for the pathogen.
Examples of the interpretation and actions in response to findings in monitoring programs are included in Table 6
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
TABLE 6 Example of Changes in Level of Concern and Resulting Actions from Pathogen and Out-ofspecification Hygiene Indicators in Various sample Types
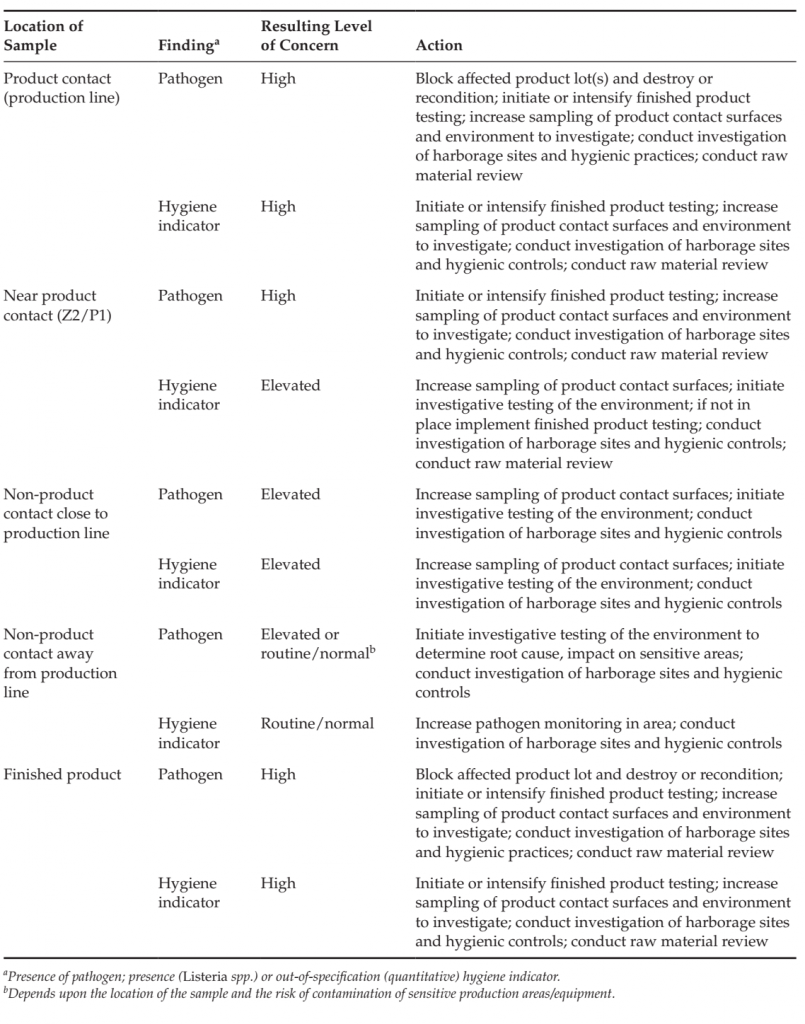
Within-specification monitoring results, along with other verification information (hygiene audits, start-up checklist, visual evaluation of cleanliness) indicate that the environment was of acceptable hygienic status on the day samples were collected. A systematic trend analysis of environmental data over time can provide greater confidence in the hygienic status of a processing line or processing area. A periodic short-term increase in a quantitative indicator such as Enterobacteriaceae could indicate that an event occurred on or prior to the sampling day impacting the hygiene of the area and facilitate a root cause investigation. Upward trends in data could indicate a gradual loss of hygienic status and enable the problem to be identified and addressed before the underlying hygiene issue leads to harborage or cross contamination with a pathogen. The results of hygiene monitoring programs should be kept in a database (e.g. Excel using pivot table functionality) facilitating the evaluation of trends and correlations in data and the generation of graphical representations and reports. Results of qualitative analyses (such as presence/absence for Salmonella, L. monocytogenes, Listeria spp.) are often documented on a factory map to facilitate the root cause analysis. Serotyping or genetic typing to identify strains of isolated pathogens is often useful to the root cause analysis. Serotyping is particularly useful for Salmonella, as there are greater than 2500 serotypes. Serotyping may also be conducted for Listeria; however, there are fewer serotypes identified and genetic typing, such as through pulsed field gel electrophoresis, may provide greater precision for a root cause investigation (Jadhav et al., 2012). Genetic typing has
also been used for Salmonella, Cronobacter spp. and other pathogens. Recurrence of the same strain in multiple sampling events, on a variety of surfaces, or following cleaning usually indicates harborage in the factory environment. The detection of different strains usually indicates transient contamination due to multiple entries into the environment from one or more routes of entry (such as through raw material or from the environment external to the factory). Data from monitoring programs communicated to the factory food safety team for review and development of corrective actions are needed. Program results may also be presented to factory personnel at operational reviews and/or through the posting of program results.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
ACCEPTANCE CRITERIA AND TESTING PROGRAMS FOR FINISHED PRODUCTS AND RAW MATERIALS
Microbiological criteria may be established for finished products, raw materials and in-process products to define the conformance of a product lot or processing line to performance objectives and to define conditions of acceptance when verification testing is conducted. Criteria may be established as requirements for products on the market or at import by regulatory agencies, as a specification by a food manufacturer for finished products or raw materials or as guidance by regulators or industry groups to food manufacturers. The utility of product testing is limited when contaminants are present at low levels and unevenly distributed. The costs of product testing are often significant due to the need to hold a corresponding product lot during the time testing is conducted. Because of such limitations, food safety management systems that incorporate preventive controls including good hygienic practices and HACCP are much more effective than a reliance on finished product testing in the absence of knowledge of such controls (ICMSF, 2002; NRC, 1985).
Although statistically limited, finished product and raw material testing may be conducted where there is limited information available about the hygienic status of a product lot (for example, a regulator’s analysis of imported product or a food producer’s analysis of raw materials). Testing may also be used for the evaluation of the suitability of finished products or raw materials where there is information from other verification activities that indicates an increased risk of contamination. The development and application of acceptance criteria for finished products and raw materials is discussed extensively by the ICMSF (2002). Lot acceptance criteria are expressed in sampling plans outlining the pathogen or indicator organism(s) of concern, the number of samples to be taken from a lot (n), the limits of acceptance (c, m and M) and the methodology to be used in verifying conformance. Sampling plans in specifications are most often defined as two-class attributes plans (acceptable and unacceptable) and three-class attributes plans (acceptable, marginally acceptable and unacceptable). Two-class attributes plans are defined by m, the level separating acceptable from unacceptable and c, the maximum allowable number of sample units yielding a result greater than m. For pathogens m is often set at 0, indicating an absence of the organism in the analytical unit tested. Three-class attributes plans are defined by m, the level separating acceptable from marginally acceptable, M, the level separating marginally acceptable from unacceptable, and c, the maximum allowable number of sample units yielding a result greater than m and less than M. If any sample is above M in a three-class plan the lot is rejected. Three-class plans are most often applied in criteria for quantitative hygienic indicator organisms as they account for variability in levels and allow identification and correction of trends before levels exceed criteria that would result in lot rejection. The ICMSF (2002) has developed standardized “cases,” sampling criteria with stringency based upon the relative risk of the microorganism or group to be analyzed and the effect of handling conditions on the relative product risk. The ICMSF has also developed representative criteria for specific product categories (ICMSF, 2011). Guidance on the sampling and shipment of finished product and raw material samples for analysis is provided in industry and regulatory guidance, including APHA (Midura and Bryant, 2001), FDA (Andrews and Hammack, 2003) and Codex Alimentarius Commission (2004).
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
MICROBIOLOGICAL MONITORING OF RAW MATERIALS
The relevance of microorganisms in raw materials is dependent upon the nature of the material, how it is processed and the material’s intended use. This will be determined in the HACCP study for the raw material. Where the microbiology of the raw material is important to the finished product microbiology, or where the microbiology of the raw material is correlated to the quality of the material (for example, sensory characteristics reflected in high plate counts), microbiological criteria are established and communicated to the vendor in specifications included with the contractual agreement (Figure 1). Such criteria indicate how a given lot of material will perform in analyses when inspected. Raw material analysis is statistically limited; the presence of a microbial pathogen or an out-of-specification hygienic indicator demonstrates that the lot was non-conforming, but the failure to isolate a pathogen does not necessarily indicate it is absent from the lot. As a result, raw material testing is most effective when it is part of an overall supplier management program that includes other verification activities, such as on-site audits, supplier
certification, evaluation of supplier performance and other inspection (such as sensory evaluation) of incoming material. When raw material monitoring is conducted for more than one operation, the program design will be based upon the most conservative use of the material. For example, a milk powder lot intended for a dry-mix operation where it will receive no microbiocidal control measure and for a wet-mix operation for a product that will be pasteurized will be analyzed by the manufacturer according to the sampling plan and risk level of the dry-mix operation.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
Establishment of Microbiological Specifications for Raw Material
Microbiological specifications for raw materials are only established when there is a specific need relative to the use of the material. It is important that specification limits established are technically attainable by the supplier through the application of HACCP and good hygienic practice. This is determined through an understanding of the nature of the raw material and how it is processed. Unrealistic specifications can lead to the use of a material that is unsuitable for its intended use even if the supplier has agreed to the specification, or to the rejection of a raw material that by its design could not meet the specification limits.
Quantitative limits in specifications may be derived from industry guidance or regulatory standards. In the absence of such standards, they are based upon an analysis of the raw material over time and from a number of operations, during normal production. Such limits must also be consistent with the expectations for finished products (as expressed in finished product specifications) and the contribution that the raw material has on the microbiological status of the finished product. Specification development should also consider those already established by the supplier; however, supplier specifications often include parameters that are not relevant to the use of the material, or do not include parameters or limits relevant to the customer need. If a raw material cannot meet expectation due to the method of manufacture of the material, it is not fit for purpose and a new material that can meet requirements should be sourced or the finished product redesigned. The stringency of microbiological specifications is based upon the risk of the material and consequences of loss of control, and on the level of confidence needed to ensure that the raw material meets microbiological requirements. Specifications should follow a standardized format, such as that outlined by the ICMSF (2002, 2011). Raw material specifications should be reviewed on an established frequency (e.g. annually) for relevance.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
Design of a Raw Material Testing Program
The scope, frequency and location of testing are determined by the raw material risk and vendor performance. Material risk is a function of the likelihood of microbial hazards inherent the materials to be present, the severity of the hazards, and how the material is used. For example, a lower risk and thus a lower sampling frequency may be assigned to a material that has robust controls, that is to be used for a product that will be cooked by the consumer, or is from a supplier with a good history of performance. A higher risk may be assigned to a raw material to be used in a product without the application of a microbiocidal process by the customer that will be used by a sensitive consumer, for a material with frequent history of failure, for a material from a new supplier, or from a supplier with poor or marginal performance. An example of a decision tree to support the classification of materials according to factors affecting raw material risk is provided in Figure 3. The risk level may determine the stringency of criteria, frequency of testing or whether a certificate of analysis (COA) will be accepted in place of testing upon receipt by the customer. An example of a raw material verification program that is adapted to raw material risk and supplier confidence is included in Table 7. Verification of the conformance of a raw material lot to specification may be conducted by the supplier and communicated in a COA, indicating through analytical testing the conformance of the specific lot to be purchased. Because testing for COA is conducted by the supplier, often at a supplier’s own laboratory, customers requiring COAs from their suppliers often conduct periodic (e.g., quarterly, biannual) testing of incoming material to verify conformance of the lot, and of the COA provided by the supplier to specification requirements. Verification testing may also be conducted by the customer as pre-shipment (i.e., before the lot has left the supplier) or upon receipt at the customer site. In the latter case, the material is blocked and is not used until the results of testing are obtained and evaluated for conformance to specification.
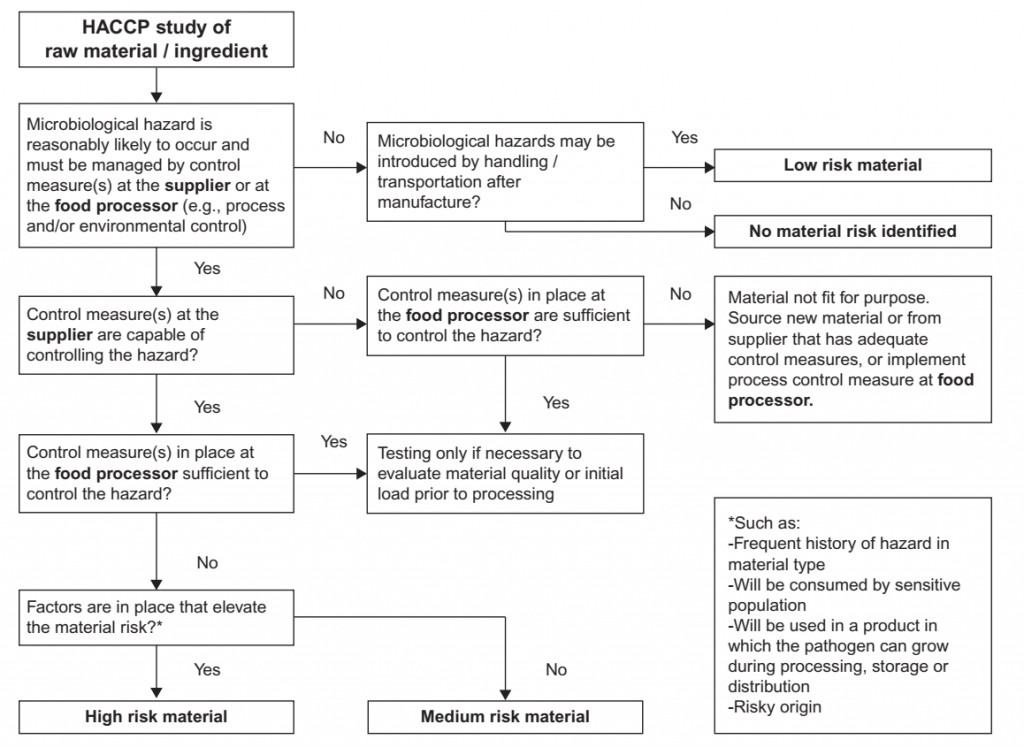
FIGURE 3 Example of a decision tree for categorizing raw material risk to determine verification activities.
Corresponding verification testing programs are outlined in Table .7.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
MICROBIOLOGICAL MONITORING OF FINISHED PRODUCTS
Finished product testing may be used to verify the overall effectiveness of a food safety system. Due to statistical limitations finished product testing cannot ensure the conformance of a lot to safety requirements and is not effective as a preventive control; however, finished product testing may be useful to evaluate the conformance of a lot to specified microbiological criteria (regulatory, customer or internal), and verify the overall effectiveness of control measures. Such testing may be conducted as within-lot or between-lot testing to demonstrate that a lot or production line is under control. Within-lot finished product testing may be conducted periodically or on each lot in response to regulatory or customer requirements. Where such testing is required as part of the contractual agreement with a customer, a COA is usually provided indicating the laboratory results. In some cases, regulators may require finished product testing on a periodic frequency. Manufacturers will design control measures and conduct their own testing more frequently to ensure that their system is able to meet regulatory criteria. The design and use of finished product monitoring is based upon a variety of factors, including:
● Sensitivity of finished product (growth, no growth, application of a lethal process);
● Exposure of product during processing (i.e. assembled, post-lethality exposed vs. in-pack pasteurization or hot fill);
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
TABLE .8 Example of the frequency of Microbiological Testing of finished Products in a Verification
Program
● Performance objective/criteria established for the finished product;
● Results of environmental monitoring or other verification of process environment hygiene;
● Risks associated with raw materials.
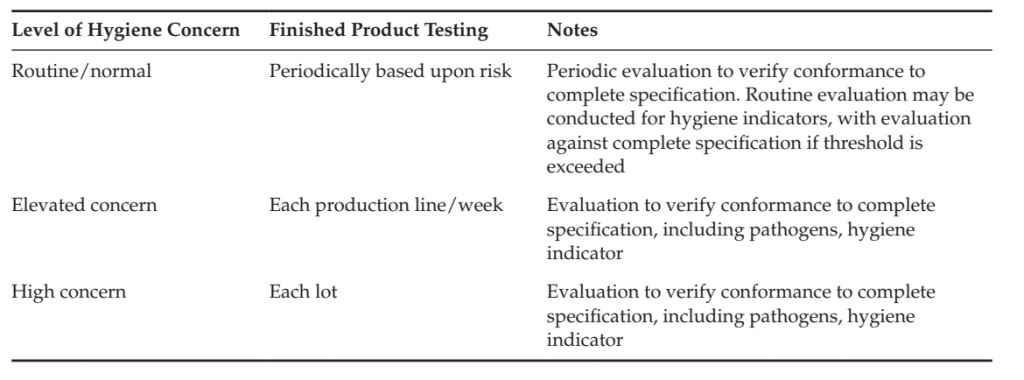
In some cases, it is practical to routinely examine each lot only for hygiene indicators, such as Enterobacteriaceae, coliforms or total plate count. Products that exceed a threshold on this initial examination are subject to evaluation in a detailed examination to evaluate conformance to complete criteria (including pathogens and indicators as defined in the finished product criteria).
Examples of the application of finished product testing are included in Table 1. The necessity and frequency of monitoring may be adapted by the level of concern of hygiene of the product and process. An example of such adjustment is included in Table .8.
Development of Microbiological Specifications for Finished Products
Finished product specifications take into account relevant regulatory or customer requirements, the hazards that may be present in raw materials and the environment, the nature of the product and process, and intended use of the material as determined in the HACCP study. Specifications include pathogens of concern as well as relevant indicator organisms, defined sampling plans and methodology. Sampling plans included in specifications should follow ICMSF format, with stringency based upon the severity of the pathogen of concern, the use of the product and the sensitivity of the consumer. Stringency may also be increased for new products or production lines, or where prior history of the product or process led to a heightened concern. Sampling plan limits for m and M should be based upon an understanding of the raw materials and processes and ideally the results of testing of products manufactured under good conditions on a variety of production days. Some regulatory authorities have established “process” criteria, which evaluate the number of positive samples as a proportion of samples collected from an operation over a period of time (ICMSF 2007). The period under evaluation is often a “moving window” of time where new results are assessed relative to a specified number of previous production days. These criteria have been applied to the analysis of pathogens in raw animal products, where control measures can reduce, but may not be able to eliminate, the presence of the pathogen of concern.
ROOT CAUSE ANALYSIS AND CORRECTIVE ACTIONS
The information collected in microbiological monitoring programs is used in conjunction with other verification activities to assess the functioning of process and environmental controls and to determine when adjustments are needed for these control measures. A positive pathogen or out-of-specification hygiene finding in the environment, raw material or finished product is a significant event and must generate an investigation, including modification of verification activities, a determination of product impact and a root cause analysis to determine what corrective actions are needed (Table 6). A positive pathogen result in a product, raw material or product contact sample cannot be negated by additional sampling unless there is confirmed evidence of a sampling or analytical error. Corresponding product will need to be destroyed or reconditioned using a process sufficient to inactivate the level of pathogenic microorganisms present in the material. A simple conclusion of a “passing contamination” with a cleaning and sanitation event followed by re-examination is not sufficient to ensure that the contamination will not recur. Root cause investigations must include a serious examination of the underlying factors and control-measure failures to ensure that appropriate corrective actions are taken and failures are not repeated. In many cases it is not possible even in an in-depth investigation to make a solid link to a specific root cause. In such situations all relevant factors that may have contributed to the contamination are addressed and monitoring programs continue with heightened stringency until there is confidence that the factors leading to the contamination have been addressed.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
References
Andrews, W.H., Hammack, T.S., 2003. Food sampling and preparation of sample homogenate. Chapter 1 in Bacteriological Analytical Manual, eighth ed. United States Food and Drug Administration, Washington, DC. <http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/ default.htm>.
Chen, Y., Scott, V.N., Freier, T.A., Kuehm, J., Moorman, M., Meyer, J., et al., 2009a. Control of Salmonella in low
moisture foods. II: hygiene practices to minimize Salmonella contamination and growth. Food Prot. Trends 29,
435–445.
Chen, Y., Scott, V.N., Freier, T.A., Kuehm, J., Moorman, M., Meyer, J., et al., 2009b. Control of Salmonella in low moisture foods. III: process validation and environmental monitoring. Food Prot. Trends 29, 493–508.
Codex Alimentarius Commission, 1997. Principles for the establishment and application of microbiological criteria
for foods. CAC/GL 21-1997. <http://www.codexalimentarius.org/>.
Codex Alimentarius Commission, 2004. General guidelines on sampling. CAC/GL 50-2004. <http://www.codexalimentarius.org/>.
Codex Alimentarius Commission, 2007a. Guidelines on the application of general principles of food hygiene to the
control of Listeria monocytogenes in foods. CAC/GL 61-2007. <http://www.codexalimentarius.org/>.
Codex Alimentarius Commission, 2007b. Principles and guidelines for the conduct of microbiological risk management (MRM). CAC/GL63-2007. <http://www.codexalimentarius.org/>.
Codex Alimentarius Commission, 2008a. Code of hygienic practice for powdered formulae for infants and young
children. CAC/RCP66-2008. <http://www.codexalimentarius.org/>.
Codex Alimentarius Commission, 2008b. Guidelines for the validation of food safety control measures. CAC/GL
69-2008. <http://www.codexalimentarius.org/>.
Cox, L.J., Keller, N., van Schothorst, M., 1988. The use and misuse of quantitative determinations of
Enterobacteriaceae in food microbiology. J. Appl. Bacteriol. (Suppl.), 237S–249S.
Duffy, J.L., Hauser, G., Hutten, H., Mager, K., Masters, K., Meesters, G.M.H., et al., 2003. Hygienic engineering of plants for the processing of dry particulate materials. European Hygienic Engineering Design Group.Document 26. <http://www.ehedg.org>.
Evancho, G.M., Sveum, W.H., Moberg, L.J., Frank, J.F., 2001. Microbiological monitoring of the food processing
environment. In: Downes, F.P., Ito, K. (Eds.), Compendium of Methods for the Microbiological Examination of
Foods American Public Health Association, Washington DC, pp. 25–35.
Grocery Manufacturers Association, 2012a. Principles for pathogen cross-contamination prevention (zoning preventative control) and zoning verification activities – environmental monitoring. GMA Submission Docket
No. FDA-2011-N-0251 20 May 2011 Industry Food Safety Practices: Informing the FDA Rule-Making Process.
Grocery Manufacturers Association, Washington, DC.
Grocery Manufacturers Association, 2012b. Product testing for verification of preventative controls. GMA
Submission Docket No. FDA-2011-N-0251 20 May 2011 Industry Food Safety Practices: Informing the FDA Rulemaking Process.
Grocery Manufacturers Association, 2010. Industry Handbook for the Safe Processing of Nuts. Grocery
Manufacturers Association, Washington, DC, <http://www.gmaonline.org/downloads/technical-guidanceand-tools/Industry_Handbook_for_Safe_Processing_of_Nuts_1st_Edition_22Feb10.pdf>.
Gorris, L.G.M., 2005. Food safety objective: an integral part of food chain management. Food Control 16, 801–809.
Holah, J., 2005. Improving zoning within food processing plants. In: Lelieveld, H.L.M., Mostert, M.A., Holah, J.
(Eds.), Handbook of Hygiene Control in the Food Industry Woodhead Publishing Ltd., Cambridge, England,
pp. 148–167.
International Commission on Microbiological Specifications for Foods (ICMSF), 2002. In: chair Tompkin, R.B. (Ed.),
Microorganisms in Foods 7. Microbiological Testing in Food Safety Management Springer, New York.
International Commission on Microbiological Specifications for Foods (ICMSF), 2011. In: chair Swanson, K.M.J.
(Ed.), Microorganisms in Foods 8. Use of Data for Assessing Process Control and Product Acceptance Springer,
New York.
International Life Sciences Institute Europe (ILSI), 2010. Impact of microbial distributions on food safety ILSI
Europe Report Series. International Life Sciences Institute Europe, Brussels, Belgium, <http://www.ilsi.org/
Europe/Publications/Microbial%20Distribution%202010.pdf>.
Jadhav, S., Bhave, M., Palombo, E.A., 2012. Methods used for the detection and subtyping of Listeria monocytogenes.
J. Microbiol. Methods 88, 327–341.
Jongenburger, I., Bassett, J., Jackson, T., Zweitering, M.H., Jewell, K., 2012a. Impact of microbial distributions on
food safety. I. Factors influencing microbial distributions and modeling aspects. Food Control 26, 601–609.
Jongenburger, I., Bassett, J., Jackson, T., Gorris, L.G.M., Jewell, K., Zweitering, M.H., 2012b. Impact of microbial
distributions on food safety. II. Quantifying impacts on public health and sampling. Food Control 26, 546–554.
Kornacki, J.L., Johnson, J.L., 2001. Enterobacteriaceae, coliforms and Escherichia coli as quality and safety indicators. In: Downes, F.P., Ito, K. (Eds.), Compendium of Methods for the Microbiological Examination of Foods
American Public Health Association, Washington, DC, pp. 69–82.
Midura, T.F., Bryant, R.G., 2001. Sampling plans, sample collection, shipment, and preparation for analysis. In:
Downes, F.P., Ito, K. (Eds.), Compendium of Methods for the Microbiological Examination of Foods American
Public Health Association, Washington, DC, pp. 13–23.
Minstry of Agriculture and Forestry, New Zealand (MAF/NZ), 2011. Guidance for the control of Listeria monocytogenes in ready-to-eat foods. Part 2. Good Operating Practice. July 2011 <www.foodsafety.govt.nz/elibrary/
industry/good-operating-practices.pdf>.
Moore, G., Griffith, C., Felding, L., 2001. A comparison of traditional and recently developed methods for monitoring surface hygiene within the food industry: a laboratory study. Dairy Food Environ. Sanit. 21, 478–488.
Motarjemi, Y., Moy, G., Risk management: application to biological hazards. Encyclopedia of Food Safety. Elsevier
(in press).
National Advisory Committee on Microbiological Criteria for Foods (NACMCF), 2006. Requisite scientific parameters for establishing the equivalence of alternative methods of pasteurization. J. Food Prot. 69, 1190–1216.
National Advisory Committee on Microbiological Criteria for Foods (NACMCF), 2010. Parameters for determining
inoculated pack/challenge study protocols. J. Food Prot. 73, 140–202.
National Fisheries Institute and National Food Processors Association (NFI/NFPA), 2002. Listeria monocytogenes
Control Manual, Draft 9. National Food Safety Initiative in 2000 project number 00-51110-9768. <http://foodscience.cornell.edu/research/labs/wiedmann/upload/SSWGLMManual.pdf>.
National Research Council (NRC) Subcommittee on Microbiological Criteria, Committee of Food Protection, Food
and Nutrition Board, 1985. Executive summary. In: An Evaluation of the Role of Microbiological Criteria for
Foods and Food Ingredients, National Academies Press, Washington, DC, pp. 1–14.
National Research Council (NRC) Committee on the Review of the Use of Scientific Criteria and Performance
Standards for Safe Food, 2003. Appendix E: international microbiological criteria. In: Scientific Criteria to
Ensure Safe Food. National Academies Press, Washington, DC, pp. 317–358.
Powell, S.C., Attwell, R.W., 1997. The use of ATP-bioluminescence as an objective measure of food hygiene standards. Int. J. Environ. Health Res. 7, 47–53.
Scott, V.N., Chen, Y., Freier, T.A., Kuehm, J., Moorman, M., Meyer, J., et al., 2009. Control of Salmonella in low moisture foods. I. Minimizing entry of Salmonella into a processing facility. Food Prot. Trends 29, 342–353.
Stringer, M., 2004. Food safety objectives – role in microbiological food safety management ILSI Europe Report
Series. International Life Sciences Institute Europe, Brussels, Belgium, <http://www.ilsi.org/Europe/
Publications/R2004Food_Safe.pdf>.
Swanson, K.M.J., Anderson, J.E., 2000. Industry perspectives on the use of microbial data for hazard analysis and
critical control point validation and verification. J. Food Prot. 63, 815–818.
Tompkin, R.B., Scott, V.N., Bernard, D.T., Sveum, W.H., Gombas, K.S., 1999. Guidelines to prevent post-processing
contamination from Listeria monocytogenes. Dairy Food Environ. Sanit. 19, 551–562.
United States Department of Agriculture, Food Safety and Inspection Service (USDA FSIS), 2012. FSIS Compliance
guideline: controlling Listeria monocytogenes in post-lethality exposed ready-to-eat meat and poultry products.
Sept. 2012 <http://www.fsis.usda.gov/PDF/Controlling_LM_RTE_guideline_0912.pdf>.
United States Food and Drug Administration (US FDA), 2008. Guidance for industry: control of Listeria monocytogenes in refrigerated or frozen ready-to-eat foods; draft guidance. Feb. 2008. United States Food and Drug
Administration, Washington, DC. <http://www.fda.gov/food/guidancecomplianceregulatoryinformation/
guidancedocuments/foodprocessinghaccp/ucm073110.htm>.
United States Food and Drug Administration (FDA), 2013. The role of testing as a verification measure in a modern
food safety system. In: Current Good Manufacturing Practice and Hazard Analysis and Risk-based Preventive
Controls for Human Food. Proposed rule, pp. 3812–3820. Fed Register 78, 3646–3824.
Van Schothorst, M., Zweitering, M.H., Ross, T., Buchanan, R.L., Cole, M.B., 2009. Relating microbiological criteria to
food safety objectives and performance objectives. Food Control 20, 967–979.
Whitehead, K.A., Smith, L.A., 2008. The detection of food soils and cells on stainless steel using industrial methods:
UV illumination and ATP bioluminescence. Int. J. Food Microbiol. 127, 121–128.
Zwietering, M.H., Stewart, C.M., Whiting, R.C., 2010. Validation of control measures in a food chain using the FSO
concept. Food Control 21, 1716–1722.
[ BRSM Certification is accredited for QMS ISO 9001, EMS ISO 14001, OSHMS ISO 45001, FSMS ISO 22000 and QMSMDD ISO 13485 and … ]
