Food safety and Allergen Management
Food allergy has been long recognized as a clinical phenomenon, with numerous reports in the 20th century medical literature (Prausnitz and Küstner, 1921; Loveless, 1950). However, while it was known that patients could suffer extremely severe and sometimes fatal reactions following ingestion of minute amounts of the offending food, food allergy was perceived as a problem for the individual sufferers alone and their clinicians. In the last decade of the 20th century this perception changed and food allergy is now recognized as an important public health problem. A major factor in this increased concern is probably the rise in the prevalence of atopic disease (Lewis et al., 1996) of which IgE-mediated food allergy can be considered a manifestation. The prevalence and incidence of food allergy and the number of severe reactions (Venter and Arshad, 2011) appears be increasing, although the lack of sound baseline epidemiological data precludes firm conclusions. The new perception of food allergy has been accompanied by the recognition that the solution to the problem lies with collaboration between all the stakeholders, including those with a food allergy and those who look after them, clinicians, public authorities and the food industry.
Many factors influence the development of allergy to common foods. However, these are outside the scope of this topic, which is concerned with the elicitation of reactions in people who already have an allergy. In this context, the ultimate aim for all stakeholders is to prevent people with food allergy reacting to the allergens to which they are sensitized. This can be achieved in two ways. One is to ensure accurate allergen declaration through labeling, so that sufferers can avoid the relevant foods. The other is to ensure that where a specific allergen is present inadvertently, for instance through cross-contact, the product does not contain it in an amount that would pose a risk and food allergy sufferers can assume it is safe for them. Both these requirements can only be fulfilled by detailed knowledge of the composition of products. The process of food manufacture is extremely complex. This complexity derives from several factors including material sourcing, processing, efficient use of equipment and other resources, and product formulation. Managing allergen risks requires an integrated approach, which takes into account all these factors throughout the supply chain, from ingredient suppliers through to retailers, and ultimately the consumer. Total avoidance of cross-contact and therefore absence of specific allergens from products where they are not part of the formulation is often not practicable. Managing allergen risks therefore requires an analysis of the risk arising from residual allergen, and subsequently a thorough and, wherever possible, quantitative assessment of risks. Although knowledge of minimum provoking doses for many allergens is inadequate, knowing how much allergen is present in a product is a key element in this assessment, and the subsequent management of the allergen risk.
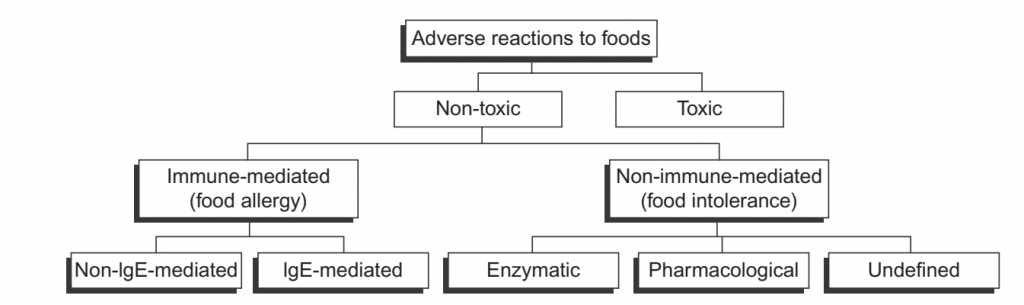
Figure-1: Classification of adverse reactions to foods by the European Academy of Allergy and Clinical Immunology.
FOOD ALLERGY: A PUBLIC HEALTH PROBLEM
Food Allergy and Food Intolerance: Food allergy forms part of a wide spectrum of adverse reactions to foods, which also includes microbial and chemical toxicity, pharmacological effects and those due to errors of metabolism, as well as idiosyncratic reactions (European Academy of Allergy & Clinical Immunology classification) (Figure.1) (Bruijnzeel-Coomen et al., 1995). Reactions which are attributable neither to toxic mechanisms nor allergy are often referred to as food intolerance, but are also frequently confused, possibly because the symptoms can often be the same. These intolerances cover a range of mechanisms, such as lactase deficiency in lactose intolerance or inborn errors of metabolism such as in galactosemia. Food allergy refers to a condition where an individual has generated an immune response to a food, and a subsequent encounter with the same food provokes an adverse (allergic) reaction. Foods can produce many different types of immune and allergic responses, but the public health concern lies largely with those in which formation of IgE antibodies to proteins in the food occurs, which are then implicated in immediate-type reactions on subsequent exposure. Allergic reactions mediated by IgE can vary from very slight, indeed barely perceptible to severe and occasionally fatal, depending on the dose, the individual and other factors. Data on the number of allergic reactions to food, and more importantly their severity, are scarce. Sampson (2005) cites a figure of 200 deaths and 30,000 emergency room (ER) visits for the USA, while a recent survey of ER in a representative sample of US hospitals estimated 125,000 reactions per annum, of which approximately 14,000 were due to anaphylaxis, the most severe and potentially lethal manifestation of food allergy (Ross et al., 2008). Allergenic foods most frequently responsible for severe and fatal reactions include peanuts, tree nuts, cows’ milk and hens’ eggs in all regions where such data are collected (Worm et al., 2010).
Food allergy affects a higher proportion of children than adults (Sampson, 2005) and reactivity to some allergenic foods, such as milk and egg, tends to be largely outgrown, while allergy to others, such as peanuts, generally persists (Venter and Arshad, 2011). Little is known about why allergy to certain foods develops, although exposure and its pattern, the characteristics of the implicated proteins, but also individual characteristics, such as atopy, all play a role. The range of minimum doses required to elicit a reaction in allergic people spans at least six orders of magnitude. Until recently, the distribution of these doses remained uncharacterized, making risk assessments arduous and fraught with uncertainty (Taylor et al., 2002; EFSA, 2004; Threshold Working Group, 2008; Crevel et al., 2008).
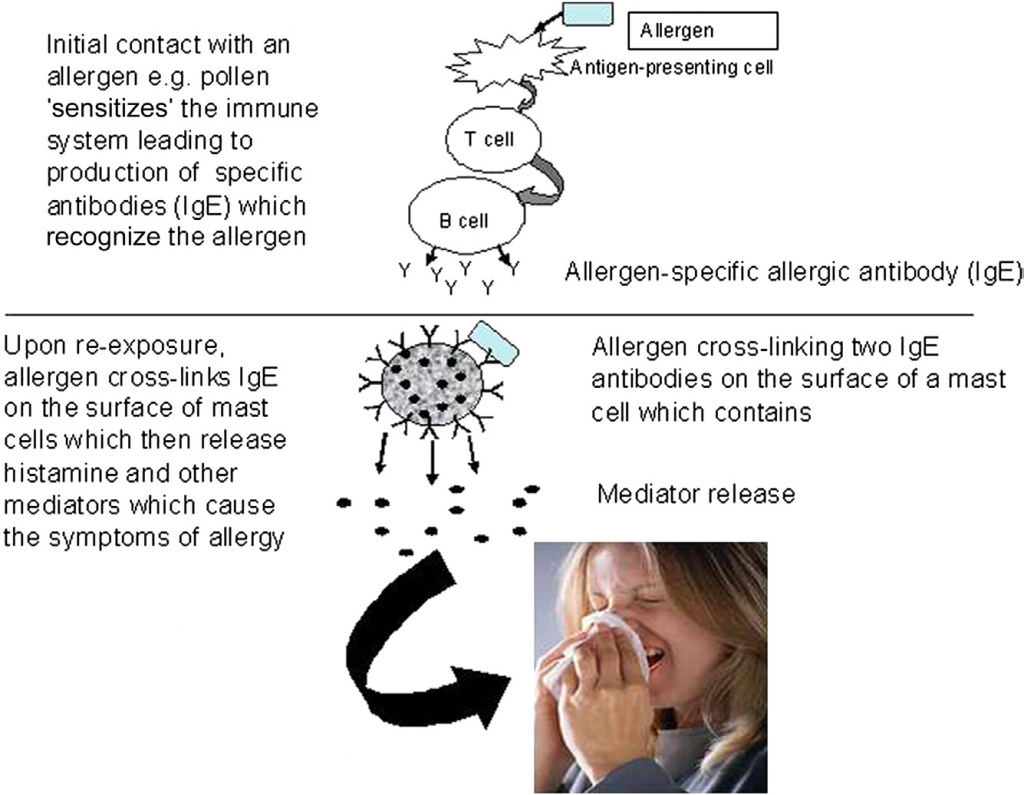
FIGURE -2 Mechanisms of food allergy.
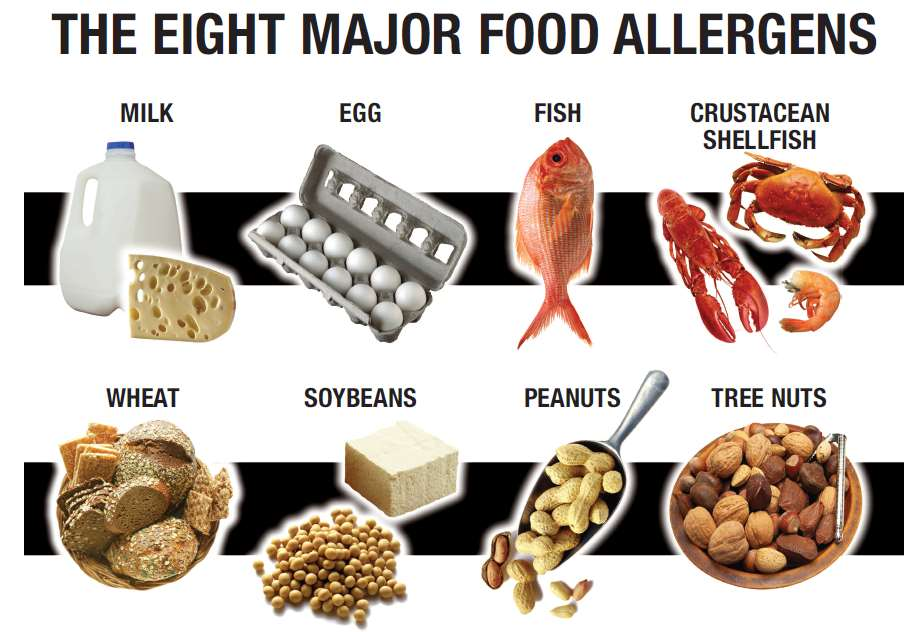
FIGURE -3 The Eight Major Food Allergens.
Symptoms of Food Allergy
The symptoms of an IgE-mediated reaction reflect directly the inflammatory response to the chemicals released from cells such as mast cells. They can affect one or more organ systems, including the skin, the gastrointestinal tract, the respiratory and cardiovascular system, with skin reactions being among the most frequently implicated. Symptoms range from pruritis or tingling in the mouth, which would not be perceptible other than to the allergic person, through eczema and rashes, angioedema, shortness of breath to the drop in blood pressure and cardiovascular collapse characteristic of anaphylactic shock.
Gastrointestinal symptoms include stomach cramps, nausea, vomiting and diarrhea. Severe allergic reactions to foods can be fatal, but information about the doses implicated in such reactions is very limited and problematic to interpret because of the circumstances under which it is generated. Recently Wainstein et al. (2010) showed that a dose of peanut as small as 20mg (5 mg peanut protein) could result in anaphylaxis, but this occurred in the clinic under controlled conditions.
While celiac disease may mimic some of the symptoms of food allergy, the underlying mechanisms are very different, as is the timing of reactions after consumption of gluten. Symptoms thus include diarrhea, bloating, abdominal pain, weight loss, failure to grow at the expected rate and malnutrition. In adults, fatigue is common. The speed with which symptoms occur after ingestion depends to some extent on the dose, but reactions are never of the rapid and catastrophic type like anaphylaxis that are associated with IgE-mediated allergies.
Prevalence of Food Allergy
One reason why food allergens need to be managed is that they constitute a threat to public health. One aspect of this threat is the potential severity of reactions and consequences for the quality of life of sufferers, but another is its prevalence in populations. Until recently, estimates of the prevalence of food allergy as a whole and allergy to individual foods were scarce and provided an inadequate basis for risk assessment and management (Rona et al., 2007). One particular problem was the considerable overestimate arising from self-reporting compared to formal diagnosis by food challenge. However, recent studies in several regions and continents, including Europe, the United States and Australia, have provided high-quality data. Thus a cross-sectional study in over 40,000 children (up to 18 years) by Gupta et al. (2011) in the USA indicated an overall prevalence of 8%, of which about 40% reported having experienced a severe reaction. Peanut, milk and shellfish were the foods implicated most frequently. Osborne et al. (2011) demonstrated that over 10% of infants up to 1 year old in Australia suffered from a challenge-verified food allergy. Countries with emerging economies also show similar trends. Kim et al. (2011) estimated that 5.3% of a birth cohort of Korean infants suffered from a food allergy, while Hu et al. (2010) observed a rise from 3.5 to 7.7% in challenge-verified food allergy from 1999 to 2009 in cross-sectional studies of infants up to 2 years old in Chongqing (China). Thus, as the social and environmental changes seen in Europe and the USA spread to other parts of the world, they will likely start to experience similar increases in the prevalence of food allergies, as seen for instance in Hong Kong and Singapore. Despite the recent promising news about specific immunotherapy for food allergens, avoidance remains the primary means whereby allergic consumers protect themselves. This requires that they know that the allergen is present (labeling) or its presence must be reduced to the point where it poses a negligible risk, hence the importance of defining minimum eliciting doses and their distribution in populations. Celiac disease was long thought to be rather rare, but recent studies indicate that it may affect over 1% of the population (Bingley et al., 2004; Lamireau and Clouzeau, 2011), but much of it is undiagnosed.
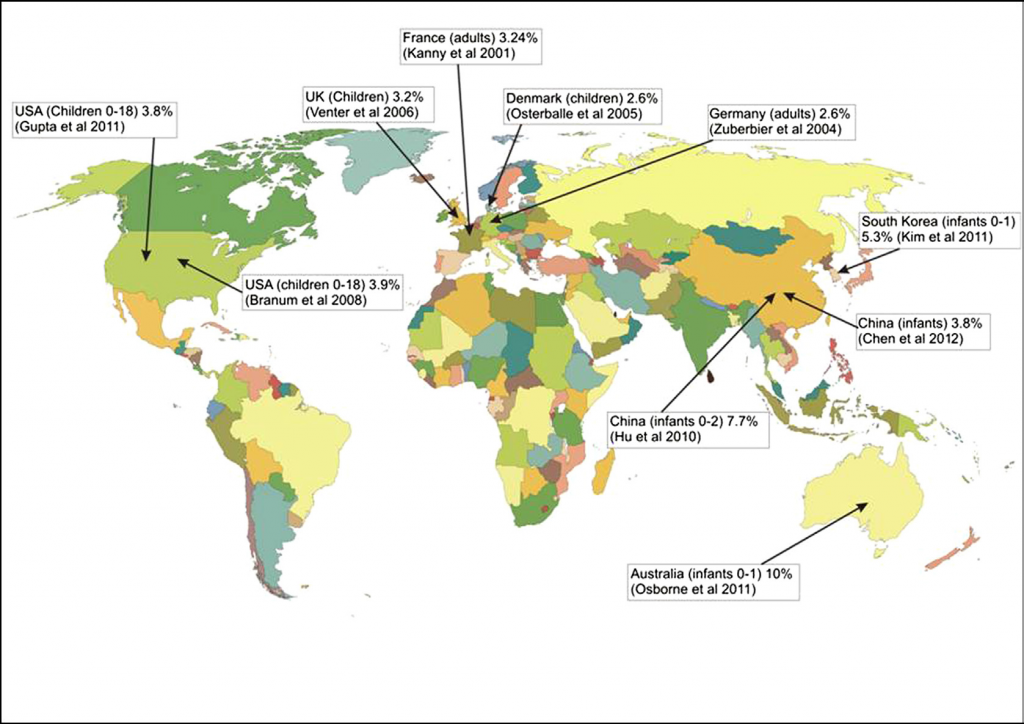
FIGURE.4 Prevalence of food allergy across the world.
allergy. Countries with emerging economies also show similar trends. Kim et al. (2011) estimated that 5.3% of a birth cohort of Korean infants suffered from a food allergy, while Hu et al. (2010) observed a rise from 3.5 to 7.7% in challenge-verified food allergy from 1999 to 2009 in cross-sectional studies of infants up to 2 years old in Chongqing (China). Thus, as the social and environmental changes seen in Europe and the USA spread to other parts of the world, they will likely start to experience similar increases in the prevalence of food allergies, as seen for instance in Hong Kong and Singapore. Despite the recent promising news about specific immunotherapy for food allergens, avoidance remains the primary means whereby allergic consumers protect themselves. This requires that they know that the allergen is present (labeling) or its presence must be reduced to the point where it poses a negligible risk, hence the importance of defining minimum eliciting doses and their distribution in populations. Celiac disease was long thought to be rather rare, but recent studies indicate that it may affect over 1% of the population (Bingley et al., 2004; Lamireau and Clouzeau, 2011), but much of it is undiagnosed.
ALLERGENIC FOODS OF PUBLIC HEALTH IMPORTANCE
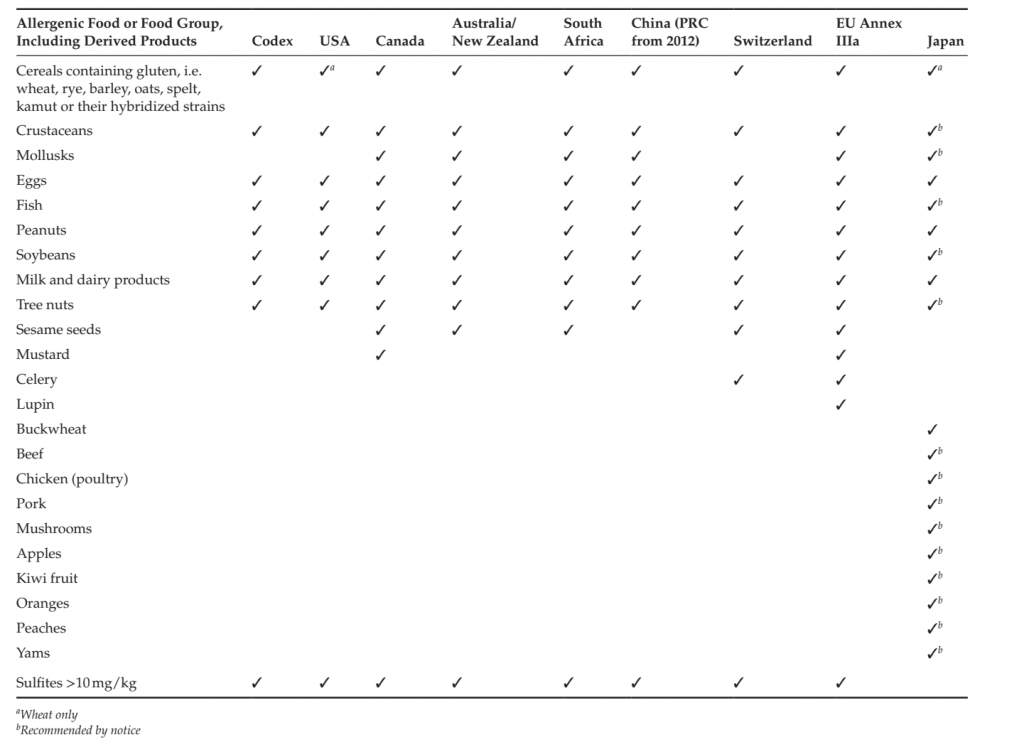
Evolution of Regulatory Allergen Lists across the World Over 160 foods have been reported to provoke allergic reactions (Hefle et al., 1996), but far fewer are considered to be of sufficient public health importance that they must be specifically managed.
Prioritization of allergenic foods as a function of their public health importance began in earnest with the FAO-WHO Expert Consultation on Food Allergies of 1995 (FAO-WHO, 1995), which identified eight major foods or food groups associated with the vast majority of allergic reactions (over 90%). This list was adopted in 1999 into the Codex General Standard on Labelling. Although the participants had rather scarce data upon which to base their conclusions, this Codex list still remains the foundation of most national and supranational regulatory allergen lists. Since then, allergen lists have been promulgated in countries covering over half the world population (Table 4.1). Identification of new allergenic foods of public health importance continues, notably through systematic epidemiological studies in projects such as Europrevall, so the lists are likely to get longer with time. Mandatory declaration of allergenic ingredients required by labeling legislation is, however, but the first and perhaps most visible consequence of priority allergen lists. More significant from an industry point of view is the implication that these priority allergens need to be actively managed to ensure that people suffering from allergies to them are not placed at risk. Inclusion of foods on such lists and additions to them therefore requires careful consideration of the benefits in terms of public health and needs to be based on sound scientific criteria, as discussed by Bjorksten et al. (2008) and van Bilsen et al. (2011). Such an approach ensures that the prioritization reflected by the lists is not diluted by inclusion of foods which pose only a relatively insignificant risk to public health. Legal/Regulatory Aspects Management of allergens starts with compliance with the regulatory requirements of the country where the product is sold, but at minimum the key allergens identified by Codex (Table 4.1). The requirement to manage allergens falls within the general ambit of food safety, which has been defined by recent standards and regulations. For instance, the Codex Alimentarius defines food safety as the concept that food will not cause harm to the consumer when it is prepared and/or eaten according to its intended use. In this definition, it notes that food safety refers specifically to the occurrence of food hazards and does not include adverse effects that may result from nutritional considerations, in other words nutritional imbalances. According to the Codex Alimentarius Commission (2003), “food safety refers to all those hazards, whether chronic or acute, that may make food injurious to the health of the consumer” and “is not negotiable.” The European Union’s Food Law (Regulation (EC) 178/2002) elaborates the concept further. Under the Regulation, food is deemed unsafe if it is either injurious to human health or unfit for human consumption. In line with the EU White Paper on food safety, it adopts a risk-based approach, recognizing that safety is not an absolute condition. This concept mirrors the criterion of “reasonable certainty of no harm” used in US law (FQPA, 1996). Thus, in determining whether a food is unsafe, the Regulation requires that two key characteristics are taken into account: the normal conditions of use of the food by food operators as well as by the final consumer, and the information provided about the food, for instance by labeling, but also more generally.
The regulation also provides guidance on how to determine whether any food is injurious to health. This includes consideration of the probable immediate and/or short-term and/or long-term effects of that food on the health of a person consuming it, but also the particular health sensitivities of a specific category of consumers where the food is intended for that category of consumers. The guidance agreed by the EU’s Standing Committee on the Food Chain and Animal Health indicates that the presence of traces of an allergen (for instance, by cross-contact) does not automatically make a food injurious to health, unless
that food had specifically been made for consumers with allergies. Contemporary approaches to food safety emphasize the need for a comprehensive integrated process (Codex Alimentarius Commission, 2003; Regulation (EC) 178/2002) both in individual food businesses and along the whole food chain. This approach follows logically from the observation that food hazards may arise at any point along that chain and highlights the need for good communication at all levels, as well as traceability.
TABLE 1. Main Regulatory Allergen Lists across the World
MANAGEMENT OF FOOD ALLERGENS
Allergens continue to form a major cause of alerts and recalls, despite legislation being now well established. For instance, in 2011, 114 allergen incidents generated 59 alerts by the UK Food Standards Agency out of a total of 105 alerts issued. Major contributors to those alerts were incorrect labeling and cross-contact. Labeling issues related either to the mandatory information (e.g. allergen not listed in ingredient list despite being deliberately added) or to incorrect precautionary labeling, highlighting the fact that if such voluntary labeling is used, it must be correct and not misleading. Cross-contact issues illustrate that minimizing the unintended presence of allergenic constituents remains a challenge for the food industry. That allergens pose a threat to public health and must therefore be managed is now beyond argument. Clearly, it is important that in addressing the risks arising from allergens, new risks are not created, which may affect even more people. Thus a key aspect of allergen management is the need to integrate it into general food safety management. An integrated system is likely to be inherently more efficient, but it is also absolutely required because the measures required to deal with one safety hazard, e.g. microbiological, may conflict with those needed to mitigate another, e.g. allergens. Thus wet cleaning is generally extremely effective in reducing allergen contamination, but can lead to severe microbiological problems in dry mix systems. However, more fundamentally, allergens differ from other contaminants with consequences for their management. Unlike those contaminants, allergens can generally be consumed safely by the vast majority of the population in any reasonable quantity and many are also important sources of nutrients, and may also have important functional attributes.
The Practice of Allergen Management
Allergen management implies actively dealing with allergens when making food products so that allergic consumers can make safe choices. This goes well beyond just avoiding the use of allergens or telling the consumer that a product may contain a particular allergen or allergens. Rather, it is about knowing where and what allergens are present throughout the food manufacturing process, deliberately or, perhaps even more importantly, unintentionally. It is also about assessing the residual risk if an unintended allergen cannot be completely removed from a product and communicating clearly and accurately that risk to consumers, neither exaggerating it nor playing it down, or requiring them to assess the risk themselves. Allergen management thus concerns the whole supply chain from the farm to the final consumer and requires accurate and comprehensive information about allergens from all those stages. Implementation of allergen management demands significant resources and therefore requires engagement of senior management within companies, as recognized by the Food Safety Management Standard ISO 22000.
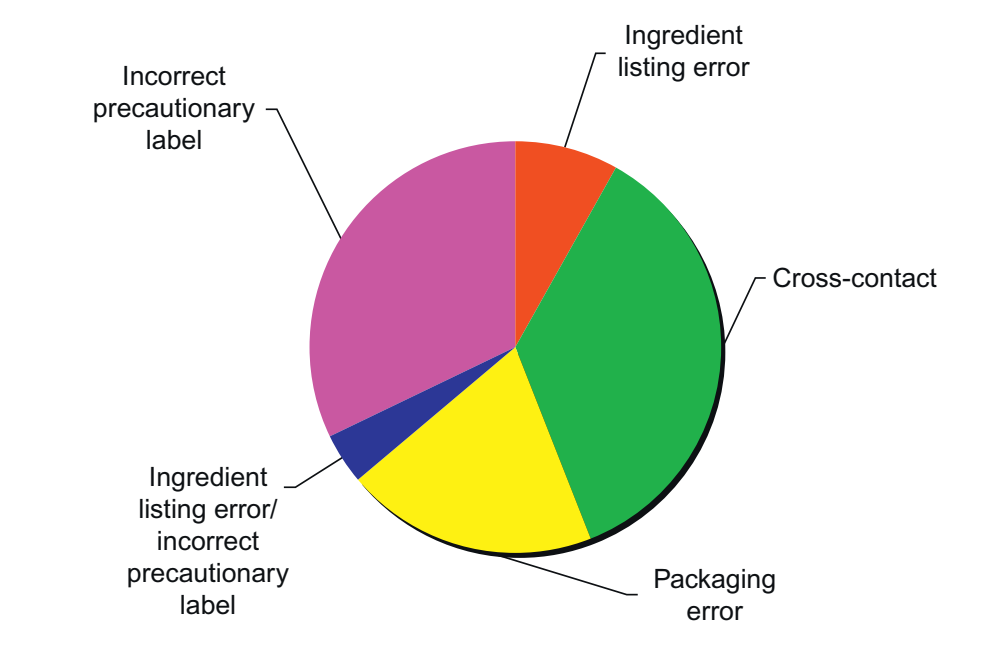
FIGURE 5 UK Food Standards Agency Allergen alerts by category in 2011.
Underlying allergen management and food safety generally are prerequisite programs which describe the basic conditions considered necessary to assure safe food production. These include considerations of premises design, hygiene, etc. Allergen management requires, first, identification of all sources of the allergen risks, then assessment of those risks and subsequently their management. This will be an iterative process, since, having identified a risk and determined that it is significant, the first step will be to look at ways of reducing it. Allergen risks can occur at all stages of the food manufacturing process, which can be summarized as Design, Sourcing, Manufacture and Delivery.
At the Design stage, key considerations include product composition and ingredient specification. Typically the allergen’s contribution to product functionality should be critically assessed and the feasibility of substitution considered. For instance, if an allergen is present as a flavor carrier, does an alternative exist which does not use that allergen or uses an allergen already present in the formulation? Ingredient specifications are also critical, particularly in respect of unintentional presence of allergens. For instance, a “gluten-free” claim would require assurance from suppliers of absence of gluten, supported by evidence that their ingredients meet appropriate specifications. Another consideration during development is what measures will be needed to manufacture the product so that no additional allergen risks are created. This requires consideration of whether a particular manufacturing site already handles a specific allergen, or at the limit, even whether the product should be made at all, since the measures needed to control allergen risks could easily make a low volume product economically unviable.
At the Sourcing stage, the critical consideration is obtaining comprehensive, accurate and reliable information about the ingredients, ensuring specifications are appropriate. Supplier questionnaires should provide information about their allergen management, including the extent to which they understand and apply processes such as HACCP (hazard analysis and critical control points). Absence of management thresholds for allergens has led many suppliers to use disclaimers or “may contain” assertions about possible allergen presence by cross-contact. Scrutiny of such disclaimers and statements may be needed to understand better the resulting risk, and quantitative information may need to be sought to permit a quantitative risk assessment. Periodic audits, either by the company’s own auditors or by auditors accredited under the major standards (e.g. BRC, IFS, GFSI) should support this process. Suppliers must also understand that they cannot change a formulation or specification without agreement or without informing their customer so that the necessary information can be conveyed to the final consumer through labeling. Inclusion of provision of appropriate (sufficient to make sound decisions about management and precautionary labeling) allergen information in contractual terms is strongly recommended. The Manufacture stage is the one over which the manufacturer has the greatest control, but possibly also the most complex. Detailed knowledge of the design and operation of the plant are imperative to successful management of allergens, particularly where it was designed before allergens were considered a food safety issue.
Cleaning also separates allergens from other components and each other, both in time and space. Indeed cleaning can also be considered as a control measure in a HACCP plan. The cleaning process, in articular if the step in the operation is a critical control point, should be subject to validation, monitoring and verification. This means that prior to manufacturing, the procedure and method of cleaning need to be validated to ensure that they are indeed effective for adequately removing allergens to the degree equired. During the manufacturing process, the cleaning process needs to be monitored periodically to ensure that it is implemented as planned. Further data collected through various verification procedures, e.g. periodic testing of final products, audits or any consumer or customer complaints, can serve to verify if the management plan is effective and implemented. Clearly, any non-compliance should prompt an investigation of the verification of the plan (i.e. is the plan implemented as planned?). If this is the case, the validation of the cleaning procedure would need to be questioned and re-evaluated. Finally, measures which can only be implemented as part of a longer-term plan include equipment and factory design. Of course, any new factory or new operations must take account of allergen management requirements at the design stage and in particular the need to minimize the presence of unintended allergen through cross-contact. Key considerations in the design are that equipment can be easily cleaned in place or that it can be dismantled. It should also avoid corners, crevices or dead-ends where material can build up and effectively form a reservoir from which it can be released into products of which it does not form part, thereby giving rise to sporadic and unpredictable cross-contact. The Delivery stage is the one at which the product is brought to the consumer. It is difficult to minimize the importance of allergen considerations at this stage and failures at this point account for a significant proportion of allergen alerts (see for example, UK Food Standards Agency reports). Critical attention to artwork is needed to ensure that the correct packaging has been used and that all allergens are listed and clear to the consumer or purchaser. At this stage the packaging should be checked for incompatible elements, such as a “dairy-free” logo, but with milk in the ingredients. In line with Codex guidelines on validating control measures, it is important to validate that the information for consumers is presented in a clear and understandable manner. Indeed, the European Union’s Regulation, 1169/2011 on Food Information for Consumers prescribes that allergens shall always be declared by reference to their common name, as defined in the Regulation, as well as being emphasized. Furthermore, the Regulation prescribes a minimum font size to improve legibility (which also applies to other ingredients). The packaging is also the vehicle for any precautionary labeling that a hazard analysis and an evaluation of the risk have shown to be required for the product; it is critical to remember in this regard that precautionary labeling can never be a substitute for good allergen management measures and does not, of itself, exonerate the manufacturer from any legal liability. If an allergen box is used, then this also needs to accord with the ingredients list – again allergy alerts, not to mention allergic reactions in consumers, have occurred as a result of discrepancies in this area.
Training
Underpinning all components of allergen management is training appropriate to the level of responsibility and role within the food business. Personnel included should range from senior management to the operatives directly involved in production and other activities. Senior management requires an understanding of the impact of food allergy on the consumer as well as on the business, and of their accountability for food safety. In contrast, those involved more directly with production and food handling activities will need guidance and instruction on procedures for minimizing cross-contact and best practice for sanitation. However, these will be most effectively conveyed if they are also placed in the wider context of food allergy. Training in allergen management is also crucial for personnel in food service establishments/catering establishments, particularly since these are well known to represent a high risk to allergic consumers. This should cover understanding and interpretation of allergen information provided by suppliers, including any precautionary labeling, as well as avoidance of cross-contact during food preparation and providing accurate information to consumers. In the European Union, provision of allergen information for non-prepacked foods, including food served by catering establishments will become mandatory under Regulation, 1169/2011.
Allergen Control Plans
Allergen control plans summarize all the necessary elements that must be checked in order to determine the allergen status of a specific facility and define the control measures that may be needed. It can thus be developed as part of the more general HACCP plan, considering the flow of materials through the factory. The allergen control plan can therefore follow the schema outlined above and should cover the following questions.
Raw Material Sourcing
● Are auditors being briefed to pay particular attention to allergen management at the supplier?
● Is the specification of raw materials and semi-finished ingredients accurate and comprehensive with regard to allergens?
● Does the specification provide enough information to assess the allergen risks accurately, given the use of the raw material?
● Have all allergenic materials that are used at the facility been identified and taken into account?
Raw Material Receipt and Storage
● Do appropriate procedures exist to assure integrity of the separation between raw materials during transport (i.e. no cross-contact during this stage)?
● Do appropriate procedures exist to ensure that raw materials are correctly assigned for storage location?
● Is storage designed to ensure segregation of allergens from other raw materials and each other and maintain it in case of failure to contain them (e.g. damage to containers)?
Manufacturing Operations
● Are material flows comprehensively described and understood, so that all possibilities for cross-contact have been identified? This should include possible reservoirs where materials can be held up and subsequently released, as well as shared pipework, etc.
● Have all operations where cross-contact can take place been identified?
● Are dedicated utensils provided where necessary for products containing food allergens?
● Have the opportunities for scheduling (e.g. non-allergen before allergen) been explored and implemented?
● Do positive measures exist to ensure that formulations are correctly made up, in particular to avoid an allergen being added by mistake?
● Is work in progress properly labeled?
● Are procedures in place to ensure that rework of products containing allergens is controlled?
● Do procedures exist to avoid mispackaging, with resulting incorrect allergen declaration?
● Do protocols exist for all cleaning operations and have they been validated?
● What measures exist to verify cleaning operations?
● Has a study to validate allergen management at the facility been conducted and documented?
● Are there procedures to avoid inadvertent introduction of allergens into manufacturing areas (e.g. on clothing, tools, etc.)?
Personnel and Training
● Have all personnel (from top management, to workers and auditors), including part-time and temporary staff, undergone training in aspects of allergen management to a level appropriate to their role?
● Is basic allergen training included in staff induction procedures appropriate to each role?
Assessing the Risk from Food Allergens
Allergy was long thought to be an area where the conventional risk assessment paradigm could not be applied. In a publication on the Threshold of Toxicological Concern, Kroes et al. (2002) acknowledge that “a particular challenge is the evaluation of food allergens and components causing other forms of intolerances, and how to determine the levels present and actual intakes vs. the limited knowledge of amounts needed for induction or elicitation of a response.” The authors in fact decided to exclude consideration of this issue from their paper. Recent work also changed this perception and demonstrated that quantitative assessment of the risk from allergens was possible. Consideration of the risk assessment paradigm revealed that the most striking gap was in the characterization of the relationship between the dose of allergen, the proportion of the allergic population that reacted to that amount and the nature of those reactions. Statistical modeling of dose distributions using data on minimum eliciting doses from food challenges has proved very successful in filling this gap for several allergens, while avoiding the difficulties of defining an absolute (population) threshold or no observed effect level (NOAEL) experimentally. The principle of this approach consists in defining the dose interval within which an individual reacts during a food challenge with the relevant allergen (Taylor et al., 2009). This interval contains this individual’s minimum eliciting dose (threshold). Thresholds from a range of individuals allergic to the same allergen are plotted against the dose as a cumulative distribution, which can then be fitted with different models for the shape of curve produced. All distributions so far have been sigmoidal when frequency of response is plotted against the logarithm of the dose and have produced good fits with the lognormal, loglogistic and Weibull models. Being able to define the distribution in this way has permitted derivation of eliciting doses corresponding to amounts of allergen predicted to cause reactions in small proportions of the allergic population (5% or less). This methodology makes use of all the available data rather than a single point and can be used to generate quantitative estimates of risk, when information about exposure is available. One of the outcomes of risk assessment can be a decision to apply a precautionary label. Under ideal circumstances, a precautionary label would result in avoidance of the product by the relevant allergic individuals. In practice and particularly in the current circumstances of extensive use of precautionary labeling, observance of the warning is far from absolute and, indeed, reaches in some cases quite low values (<50%). The reasons for this are complex, but they include the overuse previously mentioned as well as consumer confusion over the message, no doubt exacerbated by the large number of different precautionary statements (Pieretti et al., 2009). The statistical modeling approach can help to define quantitative action levels, since information about the extent of consumer compliance with precautionary labeling as a function of its prevalence can be factored in as an additional quantitative factor.
Practical Aspects of Assessing the Risk from Allergenic Ingredients
The protein component of allergenic ingredients is the determinant of allergenic risk and, as discussed above, allergic individuals react to the amount consumed on any one eating occasion (i.e. meal, snack, etc.). For any given allergenic ingredient, therefore, the starting point for assessing the risk is the protein content and the amount that will be present in a portion (or amount eaten on any one occasion). The protein content of different types of ingredient should be available from the general specification provided by the supplier, both for intended and unintended allergens. However, in the event that the supplier cannot readily provide this information, generic information is available from a variety of sources on food compositions. Taylor and colleagues summarized generally available data on several allergenic foods some years ago (Taylor et al., 2002) and the values have also been used by the US FDA Threshold Working Group (2008). Values are also available for derived ingredients such as oils derived from allergenic sources (Crevel et al., 2000). Where allergenic constituents are used as ingredients in foods (i.e. deliberately added), they are required by law to be declared irrespective of the amount present in accordance with Codex Alimentarius or regulatory requirements. The focus of risk assessment is therefore on unintended allergens present by cross-contact or otherwise. Thus the next step is to consider how much can be present by cross-contact, usually in a worst-case scenario. A typical worst-case scenario would be a product without the allergen being made on the same equipment immediately after one with a high concentration of allergen, with any already established cleaning protocol between products being used. As previously discussed, the worst-case carry-over may be subject to other constraints than allergens, such as taste, color, etc. Depending on the process and equipment, the proportion of the previous product carried over may be measured by collecting and weighing residual product in the equipment, or the allergen itself can be assayed in the following product. In some cases the proportion carried over will have been previously established or it may be sufficient initially to make a reasonable assumption based on other factors such as those already mentioned. Once a value is available for the proportion carried over, the allergenic protein concentration in the following product can be calculated, as can be the amount in a portion of the product. The amount of allergenic protein can then be compared to the amounts reported to cause reactions and a conclusion drawn about the risk posed by cross-contact.
ANALYTICAL ASPECTS OF ALLERGEN MANAGEMENT
Validation and Verification
As discussed in the preceding section, the risk arising from the unintended presence of allergens needs to be assessed. The first step in this analysis is to establish the extent of cross-contact and, if necessary, investigate different measures to reduce it. Validation is the process of checking whether or not current allergen management procedures, particularly cleaning procedures, control allergen cross-contact to an acceptable level. Verification is checking and recording that validated procedures are being implemented.
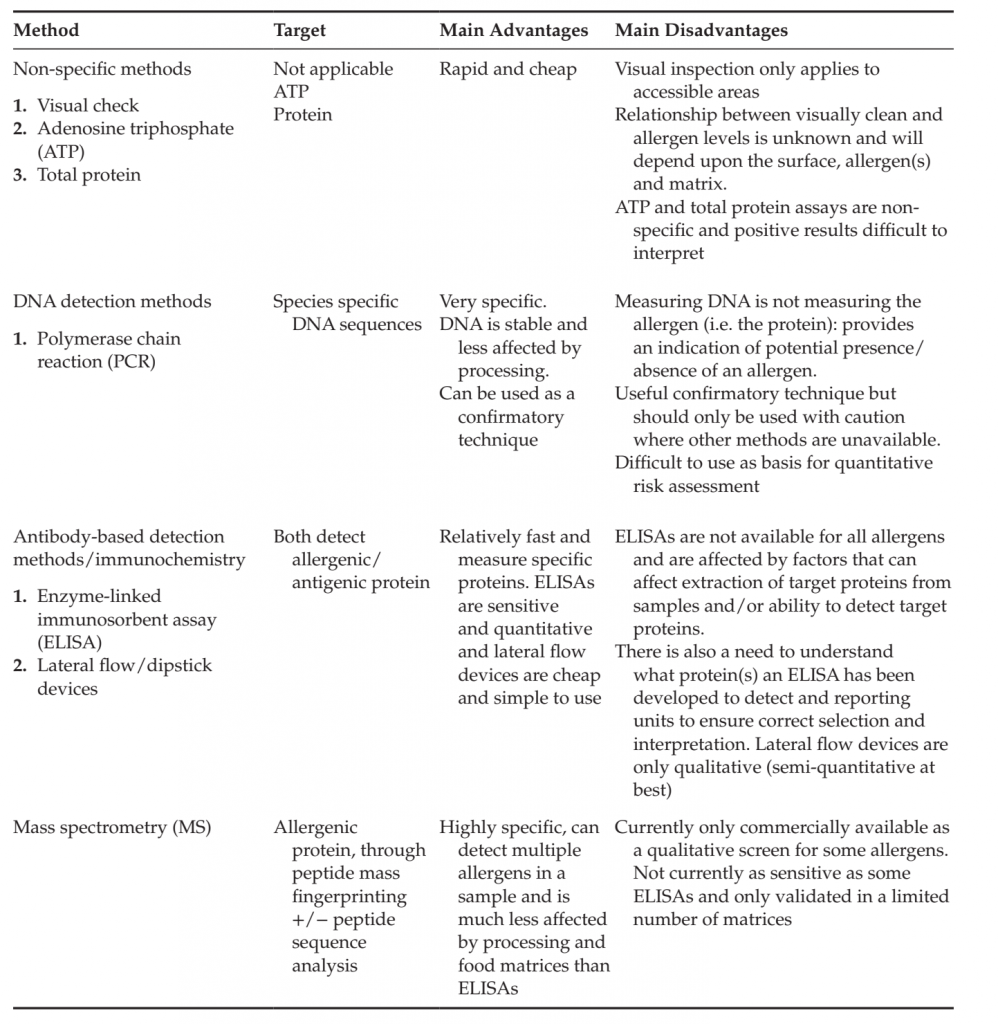
Allergen Detection Methods A variety of methods are used for allergen cleaning validation studies and the most common are listed in Table along with their major advantages and disadvantages. ELISAs are currently the most commonly used allergen detection tests and although they have a number of advantages, they also suffer from a number of disadvantages that need to be mitigated to assure the validity of the results they generate. These are covered in more detail below:
● ELISAs rely on an antibody reaction with a protein(s) and proteins exist in different forms and relative abundances in different foodstuffs. Thus, the antibodies in an ELISA may have been raised against a different mixture of proteins to those present in the potential contaminating material. Target protein(s) can also differ between ELISA kits that have the same purpose, e.g. ELISAs for milk may detect beta-lactoglobulin or casein or a mixture of milk proteins. Therefore, knowledge of the protein composition of the allergen source is required in order to ensure the correct ELISA kit is chosen to detect it and for the interpretation of the data. It is also important to understand the reporting units of the chosen ELISA (e.g. for milk, ppm beta-lactoglobulin or ppm skimmed milk powder).
● Example: if the source of potential milk protein carry-over is a whey concentrate then beta-lactoglobulin would be a suitable choice of target protein; however, if the source is skimmed milk powder then the dominant protein present would be casein. Also in the former case, reporting units in beta-lactoglobulin would be required but in the latter results could be in ppm casein or ppm skimmed milk powder.
● Food processing can alter the ability to detect an allergen, due to changes in the protein such that the antibody used in the ELISA no longer recognizes the target protein or because proteins associate with other components of the formulation and become more difficult to extract. ELISAs thus may have difficulty recognizing and produce a false negative result/reduced quantification for:
● Heated products
● Fermented products
● Hydrolyzed products
● ELISAs require the extraction of the protein into an aqueous environment prior to analysis. The efficiency of this extraction depends on the solubility of the protein(s) of interest and the formulation of the food they are to be extracted from, e.g. high fat matrices or recipes rich in polyphenols can affect extraction. To check extraction efficiency for a given sample matrix a “spike and recovery test” is recommended, e.g. if the aim is to detect skimmed milk powder in a milk-free product, then a known milk-free sample of product (e.g. prepared in the QA kitchen) can be “spiked” with a known amount of skimmed milk powder and the level of extraction quantified. The food matrix can also affect ELISAs directly, e.g. some ingredients could cross-react with the antibodies in the ELISA to give a false positive reading and others may produce colored backgrounds that need to be controlled for. Thus provision of a known allergen-free sample as a control has further value.
Whether ELISA or PCR is used, the ability to provide a reliable service will depend on the experience and expertise of the analytical laboratory with the individual allergen and tests. An experienced operator should be aware of and control for any sources of potential contamination, while carrying out the test. A certified laboratory will also ensure that all equipment is calibrated and accurate. A good laboratory should offer a confidential service and welcome, indeed even request, early discussion of the validation study providing advice on correct test selection and study design.
TABLE 2. Comparison of Analytical Methods for Allergens
Design of Validation Studies
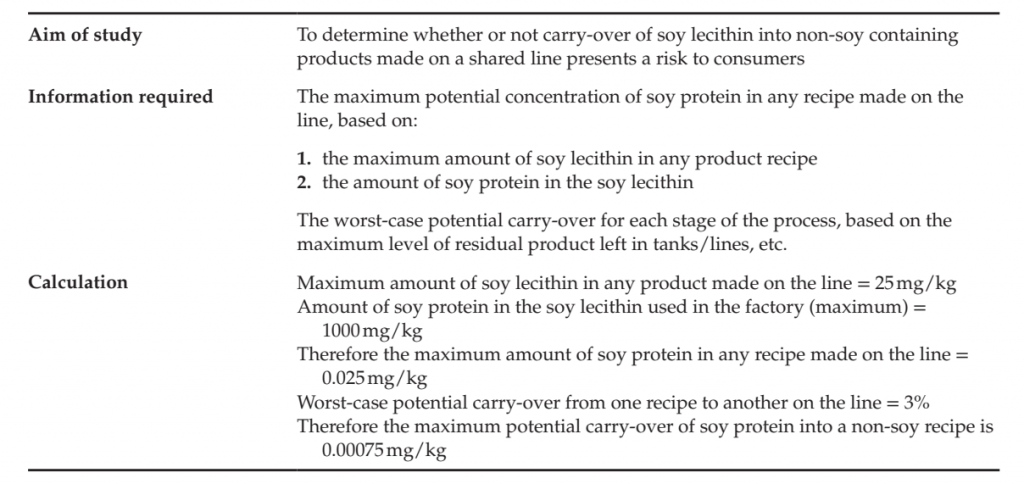
Starting with a qualitative risk assessment and then moving onto a semi-quantitative one is recommended in order to determine whether or not an analytical-based validation study is required or applicable. For example, it is sometimes possible to estimate levels of allergen carry-over from one production run to another by “worst-case scenario calculations,” i.e. measuring how much material is left behind in a process (e.g. based on film thickness on equipment or weighing brushed-out residual), what the levels of such material would be after dilution with the next product (or in the next process step), what amount of the material is allergen and therefore allergen levels in the final product that could be consumed. An example of such a calculation is described in Table. If an analytical study is required, accurate and robust analytical results are only useful if the samples analyzed have been taken as part of a correctly designed study. The aim of any validation study should be clearly defined and understood, so that the sampling procedures and subsequent analyses are correctly designed or selected and implemented. For a food product, development of a scientifically sound sampling plan includes a statistical analysis of the probability that all allergens are detected and ensures that any allergens present are accurately measured. Important sampling questions that need to be considered include whether the allergen is likely to be evenly distributed within the batch[1], the number of samples per batch that should be tested, which batches should be tested, which portion of a run should be tested, and how to obtain a specific degree of confidence (e.g. 95% confidence) that no allergen is present. The six main stages in the design of an analytical validation study are summarized in Table and an example of an analytical study is provided in Table.
TABLE: Example of a Worst-case Scenario Calculation
Verification
Cleaning processes should be periodically (e.g. yearly) verified to confirm that they remain effective and when changes are made that might impact allergen management, e.g. if there are design alterations to a process line/equipment a revalidation should be performed.
TABLE: design of a Validation Study
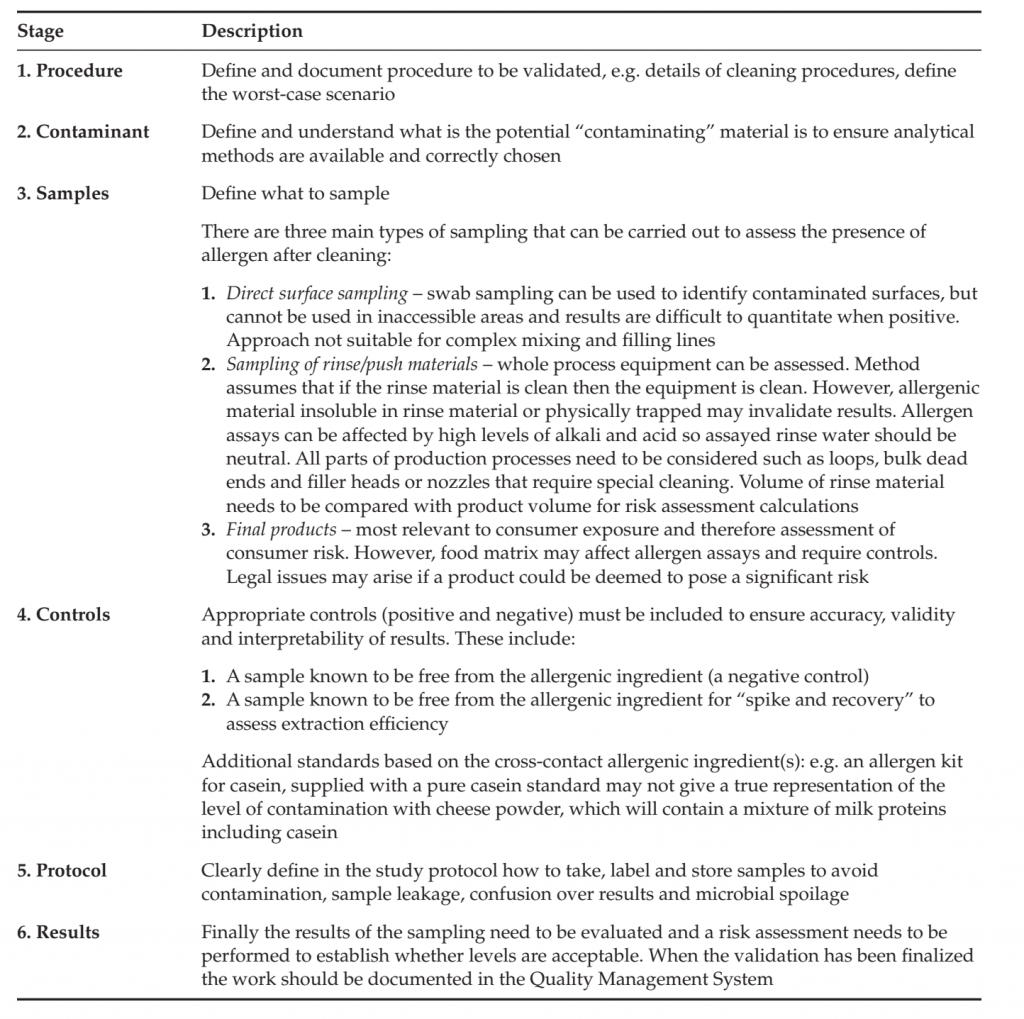
Interpretation of Validation Studies
Currently there are no agreed clinical thresholds for food allergens, although there have been some attempts in different areas of the world to provide labeling guidance. In Switzerland an action limit for labeling of 1g/kg (one part per thousand) was defined in 2001 and, as previously mentioned, in Australia and New Zealand the Allergen Bureau (an initiative of the Australian Food and Grocery Council) developed the voluntary incidental trace allergen labeling (VITAL) system, which includes a set of action levels that specify whether or not a precautionary label is required based on the level of cross-contact identified. It is clear that there is a need for agreed, acceptable limits for the labeling of non-deliberately added allergens in foods and indeed there is a great deal of time and effort currently being invested in addressing this challenge.
TABLE: Example of an Analytically-based Cleaning Validation
References
- Allergen Bureau, 2008. Voluntary Incidental Trace Allergen Labelling (VITAL). <http://www.allergenbureau.net/allergen-guide/vital/>.
- Barnett, J., Leftwich, J., Muncer, K., Grimshaw, K., Shepherd, R., Raats, M.M., et al., 2011. How do peanut and nutallergic consumers use information on the packaging to avoid allergens? Allergy 66, 969–978.
- Bingley, P.J., Williams, A.J.K., Norcross, A.J., et al., 2004. Undiagnosed coeliac disease at age seven: population based prospective birth cohort study. BMJ 328, 322–323.
- Bjorksten, B., Crevel, R., Hischenhuber, C., Lovik, M., Samuels, F., Strobel, S., et al., 2008. Criteria for identifying allergenic foods of public health importance. Regul. Toxicol. Pharmacol. 51, 42–52.
- Branum, A.M., Lukacs, S.L., 2008. NCHS data brief. Food allergy among U.S. children: trends in prevalence and hospitalizations (10), pp. 1–8.
- Bruijnzeel-Coomen, C., Ortolani, C., Aas, K., et al., 1995. EAACI position paper. Adverse reactions to food. Allergy 50, 623–635.
- Chen, J., Liao, Y., Zhang, H.Z., Zhao, H., Chen, J., Li, H.Q., et al., 2012. Jan; Prevalence of food allergy in children under 2 years of age in three cities in China.[Article in Chinese] 50 (1), pp. 5–9.
- Codex Alimentarius Commission. 2003. Principles for the risk analysis of foods derived from modern biotechnology.
- CAC/GL44-2003. Codex Alimentarius Commission, Joint FAO/WHO Food Standards Programme, Food and Agriculture Organization, Rome. (available at: <http://www.codexalimentarius.net/web/standard_list.
- do?lang=en>).
- Crevel, R.W., Kerkhoff, M.A., Koning, M.M., 2000. Allergenicity of refined vegetable oils. Food Chem. Toxicol. 38 (4), 385–393.
- Crevel, R.W., Ballmer-Weber, B.K., Holzhauser, T., Hourihane, J.O., Knulst, A.C., Mackie, A.R., et al., 2008.
- Thresholds for food allergens and their value to different stakeholders. Allergy 63 (5), 597–609.
- EFSA, 2004. European food safety authority. opinion of the scientific panel on dietitic products, nutrition and allergies on a request from the commission relating to the evaluation of allergenic foods for labelling purposes.
- EFSA J. 32, 1–197.
- FAO, 1995. Report of the FAO Technical Consultation on Food Allergies. Rome.
- Food Quality Protection Act (FQPA). 1996. Public Law 104–170 – Aug. 3, 1996, 110 STAT. 1489.
- Gupta, R.S., Springston, E.E., Warrier, M.R., Smith, B., Kumar, R., Pongracic, J., et al., 2011. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 128 (1), e9–e17.
- Hefle, S.L., Nordlee, J.A., Taylor, S.L., 1996. Allergenic foods. Crit. Rev. Food Sci. Nutr. 36 (Suppl), S69–S89.
- Hu, Y., Chen, J., Li, H., 2010. Comparison of food allergy prevalence among Chinese infants in Chongqing, 2009 versus 1999. Pediatr. Int. 52, 820–824.
- Kanny, G., Moneret-Vautrin, D.A., Flabbee, J., Beaudouin, E., Morisset, M., Thevenin, F.J., 2001. Population study of food allergy in France. Allergy Clin. Immunol. 108 (1), 133–140.
- Kim, J., Chang, E., Han, Y., Ahn, K., Lee, S.I., 2011. The incidence and risk factors of immediate type food allergy during the first year of life in Korean infants: a birth cohort study. Pediatr. Allergy Immunol. 22 (7), 715–719.
- Kroes, R., Müller, D., Lambe, J., Löwik, M.R.H., van Klaveren, J., Kleiner, J., et al., 2002. Assessment of intake from the diet. Food Chem. Toxicol. 40, 327–385.
- Lamireau, T., Clouzeau, H., 2011. Epidemiology of celiac disease. Pathol. Biol. (Paris) May 24. [Epub ahead of print].
- Lewis, S., Butland, B., Strachan, D., Bynner, J., Richards, D., Butler, N., et al., 1996. Study of the aetiology of wheezing illness at age 16 in two national British birth cohorts. Thorax 51, 670–676.
- Loveless, M.H., 1950. Milk allergy: a survey of its incidence; experiments with a masked ingestion test. J. Allergy 21, 489.
- Madsen, C.B., Hattersley, S., Buck, J., Gendel, S.M., Houben, G.F., Hourihane, J., et al., 2009. Approaches to risk
- assessment in food allergy: report from a workshop ”developing a framework for assessing the risk from allergenic foods. Food Chem. Toxicol. 47 (2), 480–489.
- Osborne, N.J., Koplin, J.J., Martin, P.E., Gurrin, L.C., Lowe, A.J., Matheson, M.C., et al., 2011. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J. Allergy Clin. Immunol. 127 (3), 668–676.
- Osterballe, M., Hansen, T.K., Mortz, C.G., Høst, A., Bindslev-Jensen, C., 2005. The prevalence of food hypersensitivity in an unselected population of children and adults. Pediatr. Allergy Immunol. 16 (7), 567–573.
- Pieretti, M.M., Chung, D., Pacenza, R., Slotkin, T., Sicherer, S.H., 2009. Audit of manufactured products: use of allergen advisory labels and identification of labeling ambiguities. J. Allergy Clin. Immunol. 124, 337–341.
- Prausnitz, C., Küstner, H., 1921. Studien über die Uberempfındlichkeit (Study of hypersensitivity). Zentralbl. Bakteriol. Mikrobiol. Hyg. Abt. Orig. 86, 160–169.
- Regulation (EU) 1169/2011 (EC) of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Official Journal of the European Union L304/18–L304/63.
- Rona, R.J., Keil, T., Summers, C., Gislason, D., Zuidmeer, L., Sodergren, E., et al., 2007. The prevalence of food allergy: a meta-analysis. J. Allergy Clin. Immunol. 120, 638–646.
- Ross, M.P., Ferguson, M., Street, D., Klontz, K., Schroeder, T., Luccioli, S., 2008. Analysis of food-allergic andanaphylactic events in the National Electronic Injury Surveillance System. J. Allergy Clin. Immunol. 121 (1), 166–171.
- Sampson, H.A., 2005. Food allergy – accurately identifying clinical reactivity. Allergy 60 (Suppl. 79), 19–24. Taylor, S.L., Hefle, S.L., Bindslev-Jensen, C., Bock, S.A., Burks, A.W., Christie, L., et al., 2002. Factors affecting the determination of threshold doses for allergenic foods: how much is too much? J. Allergy Clin. Immunol. 109, 24–30.
- Taylor, S.L., Crevel, R.W.R., Sheffield, D., Kabourek, J., Baumert, J., 2009. Threshold dose for peanut: risk characterization based upon published results from challenges of peanut-allergic individuals. Food Chem. Toxicol. 47, 1198–1204.
- Taylor, S.L., Moneret-Vautrin, D.A., Crevel, R.W., Sheffield, D., Morisset, M., Dumont, P., et al., 2010. Threshold dose for peanut: risk characterization based upon diagnostic oral challenge of a series of 286 peanut-allergic individuals. Food Chem. Toxicol. 48, 814–819.
- Threshold Working Group, 2008. Approaches to establish thresholds for major food allergens and for gluten in foods. J. Food Prot. 71, 1043–1088.
- van Bilsen, J.H., Ronsmans, S., Crevel, R.W., Rona, R.J., Przyrembel, H., Penninks, A.H., et al., 2011. Evaluation of scientific criteria for identifying allergenic foods of public health importance. Regul. Toxicol. Pharmacol. 60 (3), 281–289.
- Venter, C., Arshad, S.H., 2011. Epidemiology of food allergy. Pediatr. Clin. N. Am. 58, 327–349.
- Venter, C., Pereira, B., Grundy, J., et al., 2006. Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. J. Allergy Clin. Immunol. 117, 1118–1124.
- Wainstein, B.K., Studdert, J., Ziegler, M., Ziegler, J.B., 2010. Prediction of anaphylaxis during peanut food challenge: usefulness of the peanut skin prick test (SPT) and specific IgE level. Pediatr. Allergy Immunol. 21, 603–611.
- Worm, M., Timmermans, F., Moneret-Vautrin, A., Muraro, A., Malmheden Yman, I., Lovik, M., et al., 2010. Towards a European registry of severe allergic reactions: current status of national registries and future needs. Allergy 65, 671–680.
- Zuberbier, T., Edenharter, G., Worm, M., Ehlers, I., Reimann, S., Hantke, T., et al. 2004. Prevalence of adverse reactions to food in Germany – a population study. Allergy 59 (3), 338–345.
[1] For example is the potential carry-over from skimmed milk powder or small pieces of nut? In the former case an analytically based cleaning validation study may be suitable as the potential carry-over should be evenly spread throughout the product. However, such an approach cannot be used for sporadic contamination, as the probability of actually detecting the contamination by analytical techniques is very small and therefore non-detection only offers false reassurance. For particulate allergens a visual inspection should take place after cleaning to ensure that no particulates are left. The build-up of allergenic material on process line/equipment (e.g. heat exchange plates, vacuum equipment filters, etc.) also needs to be assessed (e.g. through regular inspection and swabs) as this can be a source of spot contamination that is very difficult to prevent and detect.
